Abstract
We show that Mn(II)-doped ZnS quantum dots coated with an acrylamide-based molecularly imprinted polymer (MIP-coated QDs) can act as a fluorescent probe for the selective and sensitive detection of the insecticide chlorpyrifos (CPF). The fluorescence of the coated QDs is quenched on loading the MIP with CPF, and the effect is much stronger for the MIP than for the non-imprinted polymer. The MIP-coated QDs were characterized by fluorescence spectrophotometry, X-ray powder diffraction, and scanning electron microscopy. Under optimal conditions, the relative fluorescence intensity of the MIP-coated QDs decreases linearly with the increasing concentration of CPF in the 0.3–60 μmol L−1 concentration range, and the detection limit is 17 nmol L−1. The method has been used for the determination of CPF in spiked water samples and gave recoveries in the range from 87.1 to 94.5 % with relative standard deviations in the 2.9 to 6.5 % range. The method is simple, safe and inexpensive.

Similar content being viewed by others
Introduction
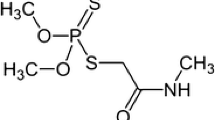
Chlorpyrifos [O, O-diethyl O-(3, 5, 6-trichloro-2-pyridyl) phosphorothioate] (CPF) is one of the most extensively applied organphosphorus insecticides, which controls a broad spectrum of insects of economically important crops [1, 2]. Due to the non-polar nature of the CPF molecule, it possesses low water solubility (≤2 μg mL−1) and readily partitions from aqueous to organic phases in the environment; therefore, the potential hazard of human exposure is high. CPF is considered a neurotoxin and endocrine disruptor [3]. CPF has been responsible for causing aquatic life toxicity in environmental water. Extensive usage of this compound leads to the accumulation of pesticide residues or their derivatives in soil, water and food.
Many methods have been described for determination of CPF including high performance liquid chromatography (HPLC) [4], gas chromatography (GC) [5, 6], liquid chromatography-mass spectrometry (LC-MS) [7], and capillary electrophoresis (CE) [8]. However, they require expensive equipment, skilled personnel and time-consuming steps of sample pretreatment [9]. Accordingly, it still remains a great challenge to develop a rapid, inexpensive and sensitive method for the detection of CPF in food and environmental analysis [10, 11].
Nowadays, quantum dots (QDs), a kind of semiconductor nanoparticles, have attracted a wide range of attention [12]. They have some unique characters, such as size tunability, narrow emission spectra but broad excitation spectra, strong signal intensity, high photo stability, and well biocompatibility [13]. The band width and maximum emission wavelength keep almost unchanged under the experimental concentration range. This is much better than the emission characteristics for typical organic dye species, which often have much broader and asymmetric emission profiles. Based on above advantages, in recent years, QDs have obtained huge development in many fields, such as biological markers [14], light-emitting diodes [15] and solar cells [16]. One major drawback that severely limits the use of common QDs (such as CdSe and CdTe) [17] in biomedical applications, particularly in the light of recent environmental regulations, is their toxicity [18].
In recent years, the emphasis has been shifted toward the fabrication of non-cadmium-based QDs. Cubic ZnS with a bulk band gap of 3.7 eV at room temperature is a common and attractive choice as a host semiconductor for producing QDs due to its low toxicity, low cost, and high stability [19]. A range of ZnS nanocrystals doped with different transition metals or rare-earth metal ions have been reported. Mn- and Cu-doped ZnS are two of the most well studied doped QDs because of their technologically suitable fluorescent properties [20]. Unfortunately, although those “direct” sensing approaches are very simple and highly sensitive, they lack an appropriate selectivity. Thus, improving selectivity of QDs based probes is very important.
Molecularly imprinted polymers (MIPs) are highly cross-linked polymers with recognition towards target molecules [21]. The synthesis of MIPs involves the formation of the template –monomer complexes through either covalent or non-covalent interactions, followed by a copolymerization with excess cross-linking agent [22]. After polymerization, the template is removed by washing with organic solvents [23]. In recent years, MIPs have attracted much attention due to their outstanding advantages, such as predetermined recognition ability, stability, relative ease and low cost of preparation, and potential application to a wide range of target molecules [24]. Combining the high selectivity of MIPs with the fluorescent properties of QDs could develop a new method for target analyte recognition [25].
In this work, the aim was to develop a novel and rapid method for determination of CPF in water samples. An eco-friendly fluorescent probe, MIP-coated QDs, was successfully fabricated by using CPF as the template molecule. The MIP-coated QDs were characterized by Fourier transformed infrared spectroscopy (FT-IR), scanning electron microscope and X-ray diffraction. The relationship between MIP-coated QDs and CPF was investigated. Then the MIP-coated QDs were used as a fluorescence probe for detection of CPF in water samples.
Experimental
Samples and reagents
The standard of CPF and acephate were purchased from Gt. Agro (Guangxi, China, http://www.gtagro.cn/). The standard of chlorpyrifos-methyl (CM) was purchased from energy chemical (Shanghai, China, http://www.energy-chemical.com/). Zinc sulfate heptahydrate (ZnSO4·7H2O) was purchased from Shuangchuan (Tianjin, China, http://www.shuangchuanchem.com/). Manganese (II) chloride tetrahydrate (MnCl2·4H2O) was purchased from Bodi (Tianjin, China, http://bdhg.company.lookchem.cn/). Sodium Sulfide (Na2S·9H2O) was purchased from Kaitong (Tianjin, China, http://www.tjkthxsj.com/). Oleic acid, ethanol, methanol, acrylamide (AM), azoisobutyronitrile (AIBN) and sodium hydroxide (NaOH) were obtained from Kermel (Tianjin, China, http://www.chemreagent.com/). Ethylene glycol dimethacrylate (EDGMA) was purchased from Aladdin (Shanghai, China, http://www.aladdin-reagent.com/). All chemicals employed in this study were of analytical grade. High-purity water was obtained from a Milli-Q water system (Millipore, Billerica, MA, USA, http://www.millipore.com/).
The standard stock solution of CPF was prepared by dissolving CPF in methanol, and the concentration was 3 mmol L−1. It was stored in a refrigerator at 4 °C.
Three river water samples were collected from Harbin (China). All water samples were stored in a refrigerator at 4 °C.
Apparatus
Fourier transform infrared (FT-IR) spectrum of the MIP-coated QDs was recorded with FT-IR360 spectrometer (Nicolet, Madison, WI, USA, http://www.artisan-scientific.com/69062.htm) using KBr method. The X-ray diffraction (XRD) spectrum was collected on a Shimadzu XRD-600 diffractometer (Kyoto, Japan, http://www.shimadzu.com.cn/) with Cu Kα radiation. The morphology of MIP-coated QDs was observed with a scanning electron microscopy (SEM, FEI Sirion, Phil-lips, Netherlands, http://www.philips.com.cn/). Fluorescence intensity studies were carried out at room temperature by using Perkin-Elmer LS-55 fluorescence spectrometer (Maryland, USA, http://www.perkinelmer.com.cn/) which was equipped with a plotter unit and a quartz cell. A KQ5200E ultrasonic apparatus (Kunshan Instrument, Kunshan, China, http://www.ks-csyq.com/) was used for making samples dispersed evenly.
Synthesis of molecularly imprinted polymer coated quantum dots
The synthesis process of MIP-coated QDs involves two major steps: the first step is the synthesis of the Mn-doped ZnS QDs, and the second one is the surface imprinting of polymers onto the oleic acid modified Mn-doped ZnS QDs.
The synthesis method of Mn-doped ZnS QDs was shown as follows. At first, 25 mmol of ZnSO4·7H2O, 2 mmol of MnCl2·4H2O and 80 mL of water were kept stirring for 20 min under the protection of nitrogen gas. Then, 10 mL, 25 mmol Na2S·9H2O solution was added dropwise into the mixture [26]. After being stirred for 30 min, 4 mL of oleic acid was added for modifying the Mn-doped ZnS QDs. Finally, the modified Mn-doped ZnS QDs was obtained after centrifugating and being washed with ethanol three times.
The synthesis method of MIP-coated QDs was carried out as follows. CPF (1 mmol), AM (4 mmol), and modified Mn-doped ZnS QDs obtained from above procedure were dispersed into 150 mL ethanol. Then AIBN (0.1 g) and the cross-linker EGDMA (10 mmol) were added. The mixture was heated at 60 °C and stirred at 300 rpm in a water bath for 24 h. Then the template was removed by Soxhlet extraction with methanol: acetic acid (19:1, v/v) until no analyte was detected. After dried in vacuum, MIP-coated QDs was obtained.
The non-molecular imprinted polymer-coated Mn-doped ZnS QDs (NIP-coated QDs) were prepared using the same procedure without addition of the template CPF.
Fluorescence analysis
Fluorescence analysis was performed on Perkin Elmer LS-55 fluorescence spectrometer. The spectra were recorded in the wavelength range of 350–750 nm upon excitation at 325 nm. Slit widths (10 nm), scan speed (200 nm min−1) and excitation voltage (750 V) were kept constant within each data set and each spectrum was the average of three scans. Quartz cell (1 cm path length) was used for all measurements.
Measurements of fluorescent response to chlorpyrifos
Thirty milligram MIP-coated QDs or NIP-coated QDs was added into a centrifuge tube, and then the given concentration of CPF solution was added. The solution pH was adjusted to 9.0 with sodium hydroxide. The constant volume was 30 mL. The fluorescence intensity was measured after fully mixing.
The river water samples were applied to evaluate the practical application. The water samples were filtered by 220 nm microporous membrane. The recovery study was carried out by spiking certain volume of CPF solution into water samples.
Results and discussion
Preparation of molecularly imprinted polymer coated quantum dots
QDs with higher fluorescence efficiencies and large specific surface areas have been widely used in biological and chemical analysis. In this work, oleic acid was used to modify the surface of Mn-doped ZnS QDs. Figure 1 illustrated the preparation process of the MIP-coated QDs. CPF was used as the template molecule. The free radical polymerization was initiated using AIBN, which could form free radicals at 60 °C [27]. AM was used as the monomer in the polymerization process because polyacrylamide was a nitrogen-containing polymer which could be solvated in aqueous medium [28]. EGDMA was added as cross-linker to freeze the polymer conformation in the right shape and orientation, which would result in specific binding sites after the removal of the template molecule. Presence of sufficient cross-linker is very important for the formation of strong polymer structure which can uphold its conformation. Different MIP-coated QDs materials were prepared by changing the template/monomer ratios (1:3; 1:4; 1:5) and the template/cross-linker ratios (1:10; 1:15; 1:20). The selectivity factor, which was calculated by the dividing the fluorescence quenching value of MIP-coated QDs by that of NIP-coated QDs, was used to compare the efficiency of different MIP-coated QDs materials. At last, 1:4 molar ratio CPF/AM and 1:10 molar ratio CPF/EGDMA were selected. The characterization of MIP-coated QDs was shown in Fig. S1 (see supplementary material).
Fluorescence study
The fluorescence intensity of the MIP-coated QDs was recorded by varying the excitation wavelength from 300 to 350 nm and was shown in Fig. S2 (see supplementary material). The weak blue peak around 444 nm was generated by the defect related to the emission of the ZnS QDs. The strong orange peak around 599 nm could be attributed to the 4 T1 → 6A1 transition of the Mn2+ impurity [20]. The maximum emission intensity at 599 nm was observed with 325 nm as the excitation wavelength. The orange fluorescence was very strong and the peak was sharp, indicating that the sizes of MIP-coated QDs were very homogeneous.
In the current work, the template CPF was entrapped in the polymer matrixes through noncovalent binding. To further elucidate the high selectivity of the MIP-coated QDs in aqueous media, the NIP-coated QDs were prepared. As shown in Fig. 2, the fluorescence intensity of MIP-coated QDs was relatively weak (spectrum c) before the removal of templates. While after Soxhlet extraction with methanol: acetic acid (19:1, v/v), the fluorescence intensity of the MIP-coated QDs was restored dramatically. However, no difference in the shape and position of the emission spectrum was observed (spectrum b). The fluorescence intensity was restored almost to that of the NIP-coated QDs (spectrum a), which indicated that the templates were removed completely from the recognition cavities in the MIP-coated QDs. It suggests that the MIP-coated QDs actually facilitate the application for the rapid and simple quantification of analytes in aqueous media.
The influences of pH and incubation time on the fluorescence intensity were investigated and the results were shown in Fig. S3 (see supplementary material).
Adsorption capacity of the composite particles
In order to investigate the binding performance of the MIP-coated QDs and NIP-coated QDs, a binding analysis was carried out using the different concentrations of CPF. The remarkable advantage of the synthesized material is a larger mass-transfer speed, where the optimal CPF adsorption time of MIP-coated QDs and NIP-coated QDs were 40 min. As shown in Fig. 3a and b, the changes in fluorescence intensity of MIP-coated QDs were much more significant than those of NIP-coated QDs, which indicated that during the MIP-coated QDs preparation process, binding sites and cavities were formed.
Selective adsorption on molecularly imprinted polymer coated quantum dots
In this work, we evaluated the effects of template molecule CPF, interference molecule acephate and analogue molecule CM on MIP-coated QDs and NIP-coated QDs. The structure of CPF, acephate and CM were shown in Fig. S4 (see supplementary material). CPF, acephate and CM are organophosphorous pesticides. The evolutions of fluorescence spectra of MIP-coated QDs or NIP-coated QDs interacted with different amounts of acephate or CM were shown in Fig. 3.
There was no obvious difference of fluorescence intensity change between the MIP-coated QDs and NIP-coated QDs for acephate. Both MIP-coated QDs and NIP-coated QDs fluorescence intensity changed by acephate are non-specific. The reason was that there was no tailor-made recognition sites formed in both MIP-coated QDs and NIP-coated QDs for acephate, so there was no significant difference on the binding capacity of the acephate.
The imprinting factors (IF) of different molecules were shown in Table S1 (see supplementary material). It indicated that the MIP-coated QDs had provided selectivity to CPF and its analogue molecule CM, which has very similar structure with the template CPF (Fig. S4). However, MIP-coated QDs provided higher selectivity to CPF (3.0) than that of CM (1.4).
Fluorescence quenching analysis
Typical fluorescence quenching of the MIP-coated QDs composites from 0.3 to 60.0 μmol L−1 CPF was studied. It demonstrated that the probe showed obvious responses to different concentration of CPF, which was very effective and suitable for practical application. The quenching in this system follows the Stern-Volmer equation:
Where the F 0 and F are the fluorescence intensities in the absence and presence of the quencher respectively, c q is the concentration of the quencher, and K sv is the Stern-Volmer constant. The quenching materials MIP-coated QDs with CPF satisfied the following equation: F 0/F = 0.0817c q + 1.0277, the correlation coefficient is 0.9992. The linear range of the calibration curve is obtained from 0.3 to 60.0 μmol L−1 with a detection limit of 17 nmol L−1 and the limit of quantification is 50 nmol L−1. When the concentration is more than 60 μmol L−1, the fluorescence quenches seriously. At this point, the fluorescence intensity of the measured value is small; the repeatability of measurement is not satisfactory.
Different analytical methods [4, 9, 11, 22, 23, 29–33] for determination of CPF were summarized briefly in Table 1. As can be seen, the recovery, precision and LOD of this method were comparable to other methods. When compared with chromatographic methods, the analytical time of this work was saved because we used fluorescence technology instead of tedious chromatographic separation process. Moreover, our method is simple, rapid and low cost when compared with other sensor methods. Furthermore, the material that we used in this method has high selectivity because of using molecularly imprinting technique.
Application to real sample analysis
In order to evaluate the feasibility of the method, three river water samples collected from Harbin (China) were analyzed. No response corresponding to CPF was observed in these water samples. Different amount of CPF with three concentrations (0.3, 3.0 and 30.0 μmol L−1) were added into the water samples. Then the water samples were processed according to the procedures described in section Measurements of fluorescent response to chlorpyrifos. The quantitative recoveries ranged from 87.1 to 94.5 %, the relative standard deviation (RSD) ranged from 2.9 to 6.5 % were obtained. The results showed that the fluorescent probe based on MIP-coated QDs has the potential applicability for CPF detection in real samples.
Conclusion
In this work, we have developed a facile strategy to fabricate the QDs-based MIP by using CPF as template and it has been successfully characterized and optimized for use as a fluorescent probe. In comparison with the antibody-or aptamer-based analytical methods, this approach is still in its infancy, and the MIP-coated QDs probe has not been widely employed. The results indicated that the MIP-coated QDs provided selectivity to CPF, which was based on the interactions of the size, shape, and functionality of the template. Furthermore, the potential advantages of this approach including simple preparation, high stability and low cost will attract more investigators for its wide applications in the near future. It is disappointing that the LOD provided by this method is inferior to some other methods. We will focus on the improvement of the sensitivity of this technique in the future.
References
Abraham J, Silambarasan S (2013) Biodegradation of chlorpyrifos and its hydrolyzing metabolite 3,5,6-trichloro-2-pyridinol by sphingobacterium. Process Biochem 48:1559–1564
Huai LF, Yang M, Liu J, Hu J (2009) Synthesis and characterization of molecularly imprinted polymer microspheres for recognition of chlorpyrifos. Chin J Appl Chem 26:1144–1148
Anirudhan TS, Alexander S (2013) Synthesis and characterization of vinyl-functionalized multiwalled carbon nanotubes based molecular imprinted polymer for the separation of chlorpyrifos from aqueous solutions. J Chem Technol Biotechnol 88:1847–1858
Ma GF, Chen LG (2014) Determination of chlorpyrifos in rice based on magnetic molecularly imprinted polymers coupled with high-performance liquid chromatography. Food Anal Methods 7:377–388
Yao ZW, Jiang GB, Liu JM, Cheng W (2001) Application of solid-phase microextraction for the determination of organophosphorous pesticides in aqueous samples by gas chromatography with flame photometric detector. Talanta 55:807–814
Ballesteros E, Parrado MJ (2004) Continuous solid-phase extraction and gas chromatographic determination of organophosphorus pesticides in natural and drinking waters. J Chromatogr A 1029:267–273
Bogialli S, Curini R, Corcia AD, Nazzari M, Samperi R (2003) A liquid chromatography—mass spectrometry assay for analyzing sulfonamide antibacterials in cattle and fish muscle tissues. Anal Chem 75:1798–1804
Hows M, Perrett D, Kay J (1997) Optimisation of a simultaneous separation of sulphonamides, dihydrofolate reductase inhibitors and β-lactam antibiotics by capillary electrophoresis. J Chromatogr A 768:97–104
Xie CG, Li HF, Li SQ, Gao S (2011) Surface molecular imprinting for chemiluminescence detection of the organophosphate pesticide chlorpyrifos. Microchim Acta 174:311–320
Xu ZX, Fang GZ, Wang S (2010) Molecularly imprinted solid phase extraction coupled to high-performance liquid chromatography for determination of trace dichlorvos residues in vegetables. Food Chem 119:845–850
Guo YR, Tian J, Liang CZ, Zhu GN, Gui WJ (2013) Multiplex bead-array competitive immunoassay for simultaneous detection of three pesticides in vegetables. Microchim Acta 180:387–395
Medintz IL, Uyeda HT, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4:435–446
Derfus AM, Chan WCW, Bhatia SNB (2004) Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 4:11–18
Sáez L, Molina J, Florea DI, Planells EM, Cabeza MC, Quintero B (2013) Characterization of l-cysteine capped CdTe quantum dots and application to test Cu(II) deficiency in biological samples from critically ill patients. Anal Chim Acta 785:111–118
Song WS, Kim JH, Yang H (2013) Silica-embedded quantum dots as downconverters of light-emitting diode and effect of silica on device operational stability. Mater Lett 111:104–107
Yang T, Lu MM, Mao XL, Liu WH, Wan L, Miao SD, Xu JZ (2013) Synthesis of CdS quantum dots (QDs) via a hot-bubbling route and co-sensitized solar cells assembly. Chem Eng J 225:776–783
Duong HD, Reddy CVG, Rhee J, Vo-Dinh T (2011) Amplification of fluorescence emission of CdSe/ZnS QDs entrapped in a sol–gel matrix, a new approach for detection of trace level of PAHs. Sensors Actuators B Chem 157:139–145
Schneider R, Wolpert C, Guilloteau H, Balan L, Lambert J, Merlin C (2009) The exposure of bacteria to CdTe-core quantum dots: the importance of surface chemistry on cytotoxicity. Nanotechnology 20:225101
Geszke-Moritz M, Clavier G, Lulek J, Schneider R (2012) Copper- or manganese-doped ZnS quantum dots as fluorescent probes for detecting folic acid in aqueous media. J Lumin 132:987–991
Bol AA, Meijerink A (2000) Doped semiconductor nanoparticles-a new class of luminescent materials? J Lumin 87–89:315–318
Stringer RC, Gangopadhyay S, Grant SA (2010) Detection of nitroaromatic explosives using a fluorescent-labeled imprinted polymer. Anal Chem 82:4015–4019
Lu Q, Chen XM, Nie L, Luo L, Jiang HJ, Chen L, Hu Q, Du SH, Zhang ZP (2010) Tuning of the vinyl groups’ spacing at surface of modified silica in preparation of high density imprinted layer-coated silica nanoparticles: a dispersive solid-phase extraction materials for chlorpyrifos. Talanta 81:959–966
Liu J, Yang M, Huai LF, Chen YT (2010) Removal of chlorpyrifos from contaminated water using molecularly imprinted polymeric microspheres. Bioinformatics and Biomedical Engineering (iCBBE), 4th International Conference on:1–4
Turiel E, Martín-Esteban A (2010) Molecularly imprinted polymers for sample preparation: a review. Anal Chim Acta 668:87–99
Carison CA, Lloyd JA, Dean SL, Walker NR, Edmiston PL (2006) Sensor for fluorene based on the incorporation of an environmentally sensitive fluorophore proximal to a molecularly imprinted binding site. Anal Chem 78:3537–3542
Liu JX, Chen H, Lin Z, Lin JM (2010) Preparation of surface imprinting polymer capped Mn-doped ZnS quantum dots and their application for chemiluminescence detection of 4-nitrophenol in tap water. Anal Chem 82:7380–7386
Narayanaswamy R, Ng SM (2006) Fluorescence sensor using a molecularly imprinted polymer as a recognition receptor for the detection of aluminium ions in aqueous media. Anal Bioanal Chem 386:1235–1244
Hawkins DM, Stevenson D, Reddy SM (2005) Investigation of protein imprinting in hydrogel-based molecularly imprinted polymers (HydroMIPs). Anal Chim Acta 542:61–65
Wei M, Zeng GY, Lu QY (2014) Determination of organophosphate pesticides using an acetylcholinesterase-based biosensor based on a boron-doped diamond electrode modified with gold nanoparticles and carbon spheres. Microchim Acta 181:121–127
Wang PP, Dai WJ, Ge L, Yan M, Ge SG, Yu JH (2013) Visible light photoelectrochemical sensor based on Au nanoparticles and molecularly imprinted poly(o-phenylenediamine)-modified TiO2 nanotubes for specific and sensitive detection chlorpyrifos. Analyst 138:939–945
Rodríguez DC, Carvajal S, Peñuela G (2013) Effect of chlorpyrifos on the inhibition of the enzyme acetylcholinesterase by cross-linking in water-supply samples and milk from dairy cattle. Talanta 111:1–7
Sanz CP, Halko R, Ferrera ZS, Rodríguez SJJ (2004) Micellar extraction of organophosphorus pesticides and their determination by liquid chromatography. Anal Chim Acta 524:265–270
Liao HT, Hsieh CJ, Chiang SY, Lin MH, Chen PC, Wu KY (2011) Simultaneous analysis of chlorpyrifos and cypermethrin in cord blood plasma by online solid-phase extraction coupled with liquid chromatography–heated electrospray ionization tandem mass spectrometry. J Chromatogr B 879:1961–1966
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (No.2572014EB06) and the National Natural Science Foundation of China (no. 21205010)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 432 kb)
Rights and permissions
About this article
Cite this article
Ren, X., Liu, H. & Chen, L. Fluorescent detection of chlorpyrifos using Mn(II)-doped ZnS quantum dots coated with a molecularly imprinted polymer. Microchim Acta 182, 193–200 (2015). https://doi.org/10.1007/s00604-014-1317-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1317-3