Abstract
Aims
GLP-1-based strategies have many advantages in treatment of type 2 diabetes mellitus (T2DM), but native GLP-1 has a short half-life in the circulation, which limits its clinical application. The purpose of this study was to evaluate the effects of GW002, a novel recombinant GLP-1 analog fusion protein produced by linking the human GLP-1 analog C-terminus to the N-terminus of human serum albumin via a linker, in vitro and in BKS-db mice.
Methods
To determine whether GW002 can activate the GLP-1 receptor in cells, the level of luciferase expression was evaluated in vitro. In vivo, body weight, food intake, non-fasting and fasting blood glucose, oral glucose tolerance test, blood glucose and insulin levels, liver histology, liver function parameters and antibody levels in BKS-db mice were investigated to evaluate the effects of GW002. Albiglutide was chosen as a positive comparator.
Results
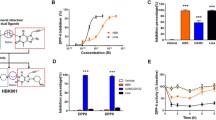
Cyclic adenosine monophosphate levels were increased in a dose-dependent manner in cells. In vivo studies demonstrated that GW002 lowers non-fasting and fasting blood glucose levels and improves glucose tolerance and insulin secretion in BKS-db mice. The degree of hepatic steatosis and hepatic biochemical indexes was also decreased. In this study, the mice body weight was not reduced significantly.
Conclusions
The above results showed that the efficacy of GW002 in BKS-db mice displayed a significant hypoglycemic effect, which indicated that GW002 might be a potential candidate for the treatment of T2DM.





Similar content being viewed by others
References
World Health Organization (2016) Global report on diabetes. ISBN 978 92 4 156525 7
Edition S (2015) Diabetes. Int Diabetes Fed. doi:10.1289/image.ehp.v119.i03
WHO (2009) Global health risks: mortality and burden of disease attributable to selected major risks. Bull World Health Organ 87:646. doi:10.2471/BLT.09.070565
Østergaard L, Frandsen CS, Madsbad S (2016) Treatment potential of the GLP-1 receptor agonists in type 2 diabetes mellitus: a review. Expert Rev Clin Pharmacol 9:241–265. doi:10.1586/17512433.2016.1121808
Wittelsberger A, Corich M, Thomas BE et al (2006) The mid-region of parathyroid hormone (1–34) serves as a functional docking domain in receptor activation. Biochemistry 45:2027–2034. doi:10.1021/bi051833a
Dong M, Li Z, Zang M et al (2003) Spatial approximation between two residues in the mid-region of secretin and the amino terminus of its receptor. Incorporation of seven sets of such constraints into a three-dimensional model of the agonist-bound secretin receptor. J Biol Chem 278:48300–48312. doi:10.1074/jbc.M309166200
Pérez Gómez A (2009) Micro tráfico de heroína, un tercer estudio realizado en Medellín y Bogotá en el 2009. Adicciones Rev Socidrogalcohol 60:470–512. doi:10.1124/pr.108.000604.470
Arulmozhi DK, Portha B (2006) GLP-1 based therapy for type 2 diabetes. Eur J Pharm Sci 28:96–108. doi:10.1016/j.ejps.2006.01.003
Schirra J, Göke B (2005) The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept 128:109–115. doi:10.1016/j.regpep.2004.06.018
Lotfy M, Singh J, Rashed H et al (2014) The effect of glucagon-like peptide-1 in the management of diabetes mellitus: cellular and molecular mechanisms. Cell Tissue Res 358:343–358. doi:10.1007/s00441-014-1959-9
Trujillo JM, Nuffer W (2014) GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy 34:1174–1186. doi:10.1002/phar.1507
Shyangdan DS, Royle P, Clar C et al (2011) Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev 71:CD006423. doi:10.1002/14651858.CD006423.pub2
Dc K, Jb B, Ll N et al (2008) Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 24:275–286. doi:10.1185/030079908X253870
Okerson T, Yan P, Stonehouse A, Brodows R (2010) Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens 23:334–339. doi:10.1038/ajh.2009.245
Scott Cohen P, Cherylyn Beckey PD (2016) Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 375:1797–1799. doi:10.1056/NEJMc1611289
Marques CMM, Motta VF, Torres TS et al (2010) Beneficial effects of exercise training (treadmill) on insulin resistance and nonalcoholic fatty liver disease in high-fat fed C57BL/6 mice. Braz J Med Biol Res 43:467–475
Salehi M, Aulinger BA, D’Alessio DA (2008) Targeting beta-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins. Endocr Rev 29:367–379. doi:10.1210/er.2007-0031
Song WJ, Seshadri M, Ashraf U et al (2011) Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab 13:308–319. doi:10.1016/j.cmet.2011.02.002
Doyle ME, McConville P, Theodorakis MJ et al (2005) In vivo biological activity of exendin (1–30). Endocrine 27:1–9. doi:10.1385/ENDO:27:1:001
Hou S, Li C, Huan Y et al (2015) Effects of E2HSA, a long-acting glucagon like peptide-1 receptor agonist, on glycemic control and beta cell function in spontaneous diabetic db/db mice. J Diabetes Res. doi:10.1155/2015/817839
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev. doi:10.1152/physrev.00034.2006
Gao Z, Bai G, Chen J et al (2009) Development, characterization, and evaluation of a fusion protein of a novel glucagon-like peptide-1 (GLP-1) analog and human serum albumin in Pichia pastoris. Biosci Biotechnol Biochem 73:688–694. doi:10.1271/bbb.80742
Mells JE, Fu PP, Sharma S et al (2012) Glp-1 analog, liraglutide, ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6 J mice fed a Western diet. Am J Physiol Gastrointest Liver Physiol 302:225–235. doi:10.1152/ajpgi.00274.2011
Ying C, Congfeng W, Xin ZOU (2013) A new long-acting GLP-1 derivative KTP ameliorates hyperglycemia and dyslipidemia and improves pancreas and fatty liver in db/db mice. Chin Sci Bull 58:2447–2453. doi:10.1007/s11434-013-5915-y
Cummings BP, Stanhope KL, Graham JL et al (2010) Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes 59:2653–2661. doi:10.2337/db09-1564
Elst D, Picha K, Parlevliet ET et al (2012) GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE* 3-Leiden mice. PLoS ONE 7(11):e49152. doi:10.1371/journal.pone.0049152
Qin X, Shen H, Liu M et al (2005) GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol 288:943–949. doi:10.1152/ajpgi.00303.2004
Baggio LL, Huang Q, Cao X, Drucker DJ (2008) An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose homeostasis. Gastroenterology 134:1137–1147. doi:10.1053/j.gastro.2008.01.017
Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M (2001) Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50:2530–2539. doi:10.2337/diabetes.50.11.2530
Conjugate P, Kim J, Baggio LL et al (2003) Development and characterization of a glucagon-like peptide 1-albumin conjugate. Diabetes 52:751–759. doi:10.2337/diabetes.52.3.751
Rolin B, Larsen MO, Gotfredsen CF et al (2002) The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab 283:E745–E752. doi:10.1152/ajpendo.00030.2002
European Medicines Agency (2014) Assessment report. 44:1–107
Shankar G, Devanarayan V, Amaravadi L et al (2008) Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal 48:1267–1281. doi:10.1016/j.jpba.2008.09.020
Bush MA, Matthews JE, De Boever EH et al (2009) Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes Metab 11:498–505. doi:10.1111/j.1463-1326.2008.00992.x
Matthews JE, Stewart MW, De Boever EH et al (2008) Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab 93:4810–4817. doi:10.1210/jc.2008-1518
Barbosa MDFS (2011) Immunogenicity of biotherapeutics in the context of developing biosimilars and biobetters. Drug Discov Today 16:345–353. doi:10.1016/j.drudis.2011.01.011
Acknowledgements
The authors would like to thank Peng Fang, Mei-Fang Ma, Qiu-Yun Du and Xiao-Bo Sheng for technical support.
Funding
Funding was provided by Ministry of Science and Technology of P. R. China (No. 2012ZX09401008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Availability of data
Some datasets generated during and analyzed during the current study are not publicly available due to the commercial secrets but are available from the corresponding author on reasonable request.
Human and animal rights
This article does not contain any studies with human subjects performed by the any of the authors.
Informed consent
None.
Additional information
Managed by Massimo Porta.
Rights and permissions
About this article
Cite this article
Ji, WW., Yu, DA., Fan, M. et al. Effects of GW002, a novel recombinant human glucagon-like peptide-1 (GLP-1) analog fusion protein, on CHO recombinant cells and BKS-db mice. Acta Diabetol 54, 685–693 (2017). https://doi.org/10.1007/s00592-017-0992-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-0992-z