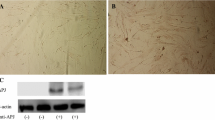
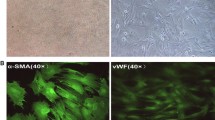
Abstract
Aims
Calcific aortic valve disease (CAVD) affects 2–6% of the population over 65 years, and age, gender, smoking, overweight, dyslipidemia, diabetes contribute to the development of this disease. CAVD results, in part, from the osteoblast differentiation of human valvular interstitial cells (VICs). This study aims to elucidate the effects of leptin on osteoblast phenotype of VICs and the signalling pathways involved.
Methods
Patients who underwent aortic valve replacement for CAVD (n = 43) were included in this study. Patients with coronary artery disease (CAD) without CAVD (n = 129) were used as controls.
Results
Patients with CAVD had higher serum leptin concentrations than CAD patients (p = 0.002). Leptin was found in calcific aortic valves, with higher concentrations in calcified versus non-calcified zones (p = 0.01). Chronic leptin stimulation of human VICs enhanced alkaline phosphatase (ALP) activity and ALP, BMP-2 and RUNX2 expression and decreased osteopontin expression. Moreover, inhibiting Akt or ERK during leptin stimulation lowered the expression of osteoblast markers in VIC.
Conclusions
Taken together, these findings indicate that leptin plays a critical role in CAVD development by promoting osteoblast differentiation of human aortic VICs in an Akt- and ERK-dependent manner. This study highlights the role of leptin in CAVD development, and further studies are needed to determine whether reducing circulating leptin levels or blocking leptin actions on VICs is efficient to slow CAVD progression.




Similar content being viewed by others
References
Nishimura RA, Otto CM, Bonow RO et al (2014) 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63(22):e57–185. doi:10.1016/j.jacc.2014.02.536
Yetkin E, Waltenberger J (2009) Molecular and cellular mechanisms of aortic stenosis. Int J Cardiol 135(1):4–13. doi:10.1016/j.ijcard.2009.03.108
Lindman BR, Clavel MA, Mathieu P et al (2016) Calcific aortic stenosis. Nat Rev Dis Primers 2:16006. doi:10.1038/nrdp.2016.6
Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH (2003) The cardiac valve interstitial cell. Int J Biochem Cell Biol 35(2):113–118
Liu AC, Joag VR, Gotlieb AI (2007) The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 171(5):1407–1418. doi:10.2353/ajpath.2007.070251
Cloyd KL, El-Hamamsy I, Boonrungsiman S et al (2012) Characterization of porcine aortic valvular interstitial cell ‘calcified’ nodules. PLoS ONE. doi:10.1371/journal.pone.0048154
Auwerx J, Staels B (1998) Leptin. Lancet 351(9104):737–742. doi:10.1016/S0140-6736(97)06348-4
Elkalioubie A, Zawadzki C, Roma-Lavisse C et al (2013) Free leptin, carotid plaque phenotype and relevance to related symptomatology: insights from the OPAL-Lille carotid endarterectomy study. Int J Cardiol 168(5):4879–4881. doi:10.1016/j.ijcard.2013.07.048
Payne GA, Kohr MC, Tune JD (2012) Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol 165(3):659–669. doi:10.1111/j.1476-5381.2011.01370.x
Singh M, Bedi US, Singh PP, Arora R, Khosla S (2010) Leptin and the clinical cardiovascular risk. Int J Cardiol 140(3):266–271. doi:10.1016/j.ijcard.2009.07.019
Zeadin M, Butcher M, Werstuck G, Khan M, Yee CK, Shaughnessy SG (2009) Effect of leptin on vascular calcification in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 29(12):2069–2075. doi:10.1161/ATVBAHA.109.195255
Zeadin MG, Butcher MK, Shaughnessy SG, Werstuck GH (2012) Leptin promotes osteoblast differentiation and mineralization of primary cultures of vascular smooth muscle cells by inhibiting glycogen synthase kinase (GSK)-3β. Biochem Biophys Res Commun 425(4):924–930. doi:10.1016/j.bbrc.2012.08.011
Ben-Zvi D, Savion N, Kolodgie F et al (2016) Local Application of Leptin Antagonist Attenuates Angiotensin II-Induced Ascending Aortic Aneurysm and Cardiac Remodeling. J Am Heart Assoc. doi:10.1161/JAHA.116.003474
Breyne J, Juthier F, Corseaux D et al (2010) Atherosclerotic-like process in aortic stenosis: activation of the tissue factor-thrombin pathway and potential role through osteopontin alteration. Atherosclerosis 213(2):369–376. doi:10.1016/j.atherosclerosis.2010.07.047
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159. doi:10.1006/abio.1987.9999
Revillion F, Charlier M, Lhotellier V et al (2006) Messenger RNA expression of leptin and leptin receptors and their prognostic value in 322 human primary breast cancers. Clin Cancer Res Off J Am Assoc Cancer Res 12(7 Pt 1):2088–2094. doi:10.1158/1078-0432.CCR-05-1904
Glader CA, Birgander LS, Söderberg S et al (2003) Lipoprotein(a), Chlamydia pneumoniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur Heart J 24(2):198–208
Shanker J, Rao VS, Ravindran V, Dhanalakshmi B, Hebbagodi S, Kakkar V (2012) Relationship of adiponectin and leptin to coronary artery disease, classical cardiovascular risk factors and atherothrombotic biomarkers in the IARS cohort. Thromb Haemost 108(4):769–780. doi:10.1160/TH12-04-0263
Kolasa-Trela R, Miszalski-Jamka T, Grudzień G, Wypasek E, Kostkiewicz M (2011) Adiponectin, leptin, and resistin in patients with aortic stenosis without concomitant atherosclerotic vascular disease. Pol Arch Med Wewn 121(10):352–359
Shapiro NI, Khankin EV, Van Meurs M et al (2010) Leptin exacerbates sepsis-mediated morbidity and mortality. J Immunol 185(1):517–524. doi:10.4049/jimmunol.0903975
Lisko I, Tiainen K, Stenholm S et al (2013) Are body mass index, waist circumference and waist-to-hip ratio associated with leptin in 90-year-old people? Eur J Clin Nutr 67(4):420–422. doi:10.1038/ejcn.2013.39
Muc M, Todo-Bom A, Mota-Pinto A, Vale-Pereira S, Loureiro C (2014) Leptin and resistin in overweight patients with and without asthma. Allergol Immunopathol (Madr) 42(5):415–421. doi:10.1016/j.aller.2013.03.004
Eveborn GW, Schirmer H, Lunde P, Heggelund G, Hansen J-B, Rasmussen K (2014) Assessment of risk factors for developing incident aortic stenosis: the Tromsø Study. Eur J Epidemiol 29(8):567–575. doi:10.1007/s10654-014-9936-x
Ngo MV, Gottdiener JS, Fletcher RD, Fernicola DJ, Gersh BJ (2001) Smoking and obesity are associated with the progression of aortic stenosis. Am J Geriatr Cardiol 10(2):86–90
Katz R, Budoff MJ, Takasu J et al (2009) Relationship of metabolic syndrome with incident aortic valve calcium and aortic valve calcium progression: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes 58(4):813–819. doi:10.2337/db08-1515
Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A (2007) Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol 27(6):1259–1268. doi:10.1161/ATVBAHA.106.137851
Schroeter MR, Schneiderman J, Schumann B et al (2007) Expression of the leptin receptor in different types of vascular lesions. Histochem Cell Biol 128(4):323–333. doi:10.1007/s00418-007-0319-1
Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL (2001) Leptin enhances the calcification of vascular cells artery wall as a target of leptin. Circ Res 88(9):954–960. doi:10.1161/hh0901.090975
Kaden JJ, Bickelhaupt S, Grobholz R et al (2004) Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis 13(4):560–566
Gomez-Stallons MV, Wirrig-Schwendeman EE, Hassel KR, Conway SJ, Yutzey KE (2016) Bone morphogenetic protein signaling is required for aortic valve calcification. Arterioscler Thromb Vasc Biol 36(7):1398–1405. doi:10.1161/ATVBAHA.116.307526
Xu J-C, Wu G-H, Liu H-L, Liu J-T, Yan X-J, Chen J-T (2010) The effect of leptin on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in nude mice. Saudi Med J 31(6):615–621
Rusanescu G, Weissleder R, Aikawa E (2008) Notch signaling in cardiovascular disease and calcification. Curr Cardiol Rev 4(3):148–156. doi:10.2174/157340308785160552
Bobryshev YV, Orekhov AN, Sobenin I, Chistiakov DA (2014) Role of bone-type tissue-nonspecific alkaline phosphatase and PHOSPO1 in vascular calcification. Curr Pharm Des 20(37):5821–5828
Cho H-J, Cho H-J, Kim H-S (2009) Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep 11(3):206–213. doi:10.1007/s11883-009-0032-8
Speer MY, Chien Y-C, Quan M et al (2005) Smooth muscle cells deficient in osteopontin have enhanced susceptibility to calcification in vitro. Cardiovasc Res 66(2):324–333. doi:10.1016/j.cardiores.2005.01.023
Yuan ZS, Zhou YZ, Liao XB et al (2015) Apelin attenuates the osteoblastic differentiation of aortic valve interstitial cells via the ERK and PI3-K/Akt pathways. Amino Acids. doi:10.1007/s00726-015-2020-3
Choi YH, Kim Y-J, Jeong HM, Jin Y-H, Yeo C-Y, Lee KY (2014) Akt enhances Runx2 protein stability by regulating Smurf2 function during osteoblast differentiation. FEBS J 281(16):3656–3666. doi:10.1111/febs.12887
Liu G-Y, Liang Q-H, Cui R-R et al (2013) Leptin promotes the osteoblastic differentiation of vascular smooth muscle cells from female mice by increasing RANKL expression. Endocrinology 155(2):558–567. doi:10.1210/en.2013-1298
Chavez RJ, Haney RM, Cuadra RH et al (2012) Upregulation of thrombospondin-1 expression by leptin in vascular smooth muscle cells via JAK2- and MAPK-dependent pathways. Am J Physiol Cell Physiol 303(2):C179–C191. doi:10.1152/ajpcell.00008.2012
Acknowledgements
This work was supported by the Fondation de France and the Nouvelle Société Francophone d’Athérosclérose (NSFA). We are grateful to Bertrand Vaast and Alexandre Ung for their excellent technical assistance. Bart Staels is a member of the Institut Universitaire de France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights disclosure
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Managed by Massimo Federici.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rosa, M., Paris, C., Sottejeau, Y. et al. Leptin induces osteoblast differentiation of human valvular interstitial cells via the Akt and ERK pathways. Acta Diabetol 54, 551–560 (2017). https://doi.org/10.1007/s00592-017-0980-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-0980-3