Abstract
Aims
To improve insulin sensitivity, insulin-sensitizing drugs such as metformin are commonly used in overweight and obese T1D patients. Similarly to metformin, d-chiro-inositol (DCI), as putative mediator of intracellular insulin action, can act as insulin sensitizer. The aim of this pilot study was to evaluate the hypothesis that DCI plus folic acid may improve glucose control reducing insulin resistance in overweight or obese T1D patients.
Methods
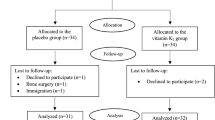
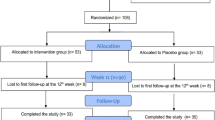
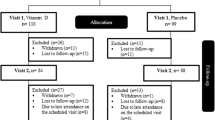
A 24-week randomized control trial was carried out in 26 overweight or obese T1D patients, undergoing intensive insulin therapy. Patients were randomized to 1 g DCI plus 400 mcg folic acid once daily (treated group) or to 400 mcg folic acid only once daily (control group). The primary end point was to evaluate the efficacy of DCI on metabolic control as assessed by HbA1c. As secondary endpoints, BMI and insulin requirement (IR) were evaluated. Paired t test (two tailed) and analysis of variance were used to evaluate differences in HbA1c, BMI and IR at different time points.
Results
A significant reduction in HbA1c levels in treated group versus control group (7.5% ± 0.9 vs. 7.9% ± 1.7, respectively, p < 0.05) was observed. However, no significant reduction in BMI and IR was observed [(BMI 25.7 ± 2.8 vs. 26.7 ± 1.0, respectively, p NS); (IR 0.52 ± 0.26 vs. 0.52 ± 0.19, respectively, p NS)].
Conclusions
This trial demonstrated for the first time that DCI plus folic acid oral supplementation can improve metabolic control in overweight T1D patients.
ClinicalTrial.Gov ID
NCT02730949.



Similar content being viewed by others
Abbreviations
- DCI:
-
d-chiro-Inositol
- T1D:
-
Type 1 diabetes
- IR:
-
Insulin requirement
- MDI:
-
Multiple daily injection
References
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Pozzilli P, Buzzetti R (2007) A new expression of diabetes: double diabetes. Trends Endocrinol Metab 18(2):52–57
Pozzilli P, Guglielmi C (2009) Double diabetes: a mixture of type 1 and type 2 diabetes in youth. Endocr Dev 14:151–166
Ortega Juan F et al (2014) Metformin does not attenuate the acute insulin-sensitizing effect of a single bout of exercise in individuals with insulin resistance. Acta Diabetol 51(5):749–755
Vella S, Buetow L et al (2010) The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 53(5):809–820
Larner J, Brautigan DL, Thorner MO (2010) d-Chiro-inositol glycans in insulin signaling and insulin resistance. Mol Med 16(11–12):543–551
Larner J (2002) d-Chiro-inositol: its functional role in insulin. Action and its deficit in insulin resistance. Int J Exp Diabetes Res 3:47–60
Ortmeyer HK, Bodkin NL, Lilley K, Larner J, Hansen BC (1993) Chiro-inositol deficiency and insulin resistance. I. Urinary excretion rate of chiro-inositol is directly associated with insulin resistance in spontaneously in diabetic rhesus monkeys. Endocrinology 132:640–645
Larner J, Craig JW (1996) Urinary myo-inositol-to chiro-inositol ratios and insulin resistance. Diabetes Care 19(1):76–78
Gluck G, Anguelova T et al (2010) Synergistic effects of d-chiro-inositol and manganese on blood glucose and body weight of streptozotocin-induced diabetic rats. Curr Bioact Compd 6:90–96
Yao Y, Shan F, Bian J et al (2008) d-Chiro-inositol-enriched tartary buckwheat bran extract lowers the blood glucose level in KK-Ay mice. J Agric Food Chem 56(21):10027–10031
Suzuki S, Kawasaki H et al (1994) Urinary chiro-inositol excretion is an index marker of insulin sensitivity in Japanese type II diabetes. Diabetes Care 17:1465–1468
Sudchada P, Saokaew S et al (2012) Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 98(1):151–158
Solini A, Santini E, Ferrannini E (2006) Effect of short-term folic acid supplementation on insulin sensitivity and inflammatory markers in overweight subjects. Int J Obes 30:1197–1202
Setola E, Monti LD et al (2004) Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: relationship between homocysteine levels and hyperinsulinemia. Eur J Endocrinol 151(4):483–489
D’Anna R, Scilipoti A et al (2013) Myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care 36(4):854–857
Cianci A, Panella M, Caruso S et al (2015) d-Chiro-inositol and alpha lipoic acid treatment of metabolic and menses disorders in women with PCOS. Gynecol Endocrinol 31(6):483–486
La Marca A, Grisendi V et al (2015) The menstrual cycle regularization following d-chiro-inositol treatment in PCOS women: a retrospective study. Gynecol Endocrinol 31(1):52–56
Laganà AS, Barbaro L, Pizzo A (2015) Evaluation of ovarian function and metabolic factors in women affected by polycystic ovary syndrome after treatment with d-chiro-inositol. Arch Gynecol Obstet 291(5):1181–1186
Nestler John E et al (1999) Ovulatory and metabolic effects of d-chiro-inositol in the polycystic ovary syndrome. N Engl J Med 340:1314–1320
Galazis N, Galazi M, Atiomo W (2011) d-Chiro-inositol and its significance in polycystic ovary syndrome: a systematic review. Gynecol Endocrinol 27(4):256–262
Ku BJ, Kim HJ, Park KS (2007) The clinical study to evaluate the safety and efficacy of d-chiro-inositol in patients with type 2 diabetes. Korean J Med 72(1):29–36
Yoo KH, Kim JH et al (2007) Effect of pinitol on glucose metabolism and adipocytokines in uncontrolled type 2 diabetes. Diabetes Res Clin Pract 77(1):S247–S251
Pozzilli P, Guglielmi C, Caprio S, Buzzetti R (2011) Obesity, autoimmunity, and double diabetes in youth. Diabetes Care 34(2):S166–S170
Dang NT, Mukai R, Yoshida K, Ashida H (2010) Pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci Biotechnol Biochem 74(5):1062–1067
Funding
This study was supported by an educational grant to University Campus Bio-Medico by LJ Pharma. LJ Pharma also provides the caps of 1 g of DCI plus 200 mcg folic acid (CHIROFOL 500 ® LJ Pharma) and the caps of 200 mcg folic acid (FOLICO ® LJ Pharma). The research sponsor played no role in the design or conduct of the study, collection, management, analysis or interpretation of the data.
Author contributions
A.R.M. designed the study, performed statistical analysis and wrote the manuscript. M.M., R.D.T., S.K., D.M. C.G., A.L.P researched data, E.F. contributed to the discussion, S.M. and P.P. reviewed/edited the manuscript. A.R.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Maurizi, A.R., Menduni, M., Del Toro, R. et al. A pilot study of d-chiro-inositol plus folic acid in overweight patients with type 1 diabetes. Acta Diabetol 54, 361–365 (2017). https://doi.org/10.1007/s00592-016-0954-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0954-x