Abstract
Aims
The RECORD study evaluated the effects of rosiglitazone on cardiovascular outcomes. A 4-year observational follow-up was added to the study to monitor the occurrence of cancer and bone fractures. We present the cancer and bone fracture data aggregated across the main study and its observational follow-up.
Methods
RECORD was a multicentre, open-label trial in people with type 2 diabetes on metformin or sulfonylurea monotherapy randomly assigned to addition of rosiglitazone (n = 2,220) or to a combination of metformin and sulfonylurea (n = 2,227). At the end of the main study, patients stopped study drug and were invited to enter the observational follow-up during which glucose-lowering treatment was selected by the patient’s physician. Serious adverse events of cancer and serious and non-serious events of bone fracture were recorded. The study is registered with ClinicalTrials.gov, number NCT00379769.
Results
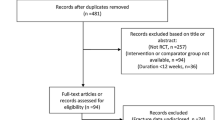
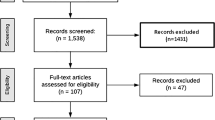
Of the 4,447 patients comprising the intent-to-treat population, 2,546 entered the observational follow-up (1,288 rosiglitazone, 1,258 metformin/sulfonylurea) and added 9,336 patient-years experience to the main RECORD study, making an aggregate of 33,744 patient-years. Based on the totality of follow-up, malignancies were reported in 179 of 2,220 patients (8.1 %) in the group originally randomised to rosiglitazone and in 195 of 2,227 patients (8.8 %) in the group allocated metformin/sulfonylurea [relative risk, RR, 0.92 (95 % CI 0.76–1.12)]. More patients reported bone fractures in the rosiglitazone group (238, 10.7 %) than in the metformin/sulfonylurea control [151, 6.8 %; RR 1.58 (1.30–1.92)]. For women, the corresponding figures were rosiglitazone 156 (14.5 %), metformin/sulfonylurea 91 (8.5 %), RR 1.71 (1.34–2.18), and for men, the corresponding figures were rosiglitazone 82 (7.2 %), metformin/sulfonylurea 60 (5.2 %), RR 1.37 (0.99–1.90). Potentially high-morbidity fractures (hip, pelvis, femur, and spine) occurred in the same number of patients (31, 1.4 %) in the two treatment groups.
Conclusions
We conclude that data from a 4-year observational follow-up, combined with the main RECORD study data, do not suggest an increased risk of cancer in patients randomised to rosiglitazone combination use compared with those randomised to metformin/sulfonylurea. Consistent with the main study, rosiglitazone is associated with an increased risk of peripheral bone fracture in women, and probably in men, but the combined data do not suggest an increase in potentially high-morbidity (hip, pelvis, femur, and spine) fractures.
Similar content being viewed by others
References
El-Hage J. http://www.fda.gov/ohrms/dockets/ac/05/slides/2005-4169s2_02_02-fda-elhage.ppt. Accessed Aug 2014
Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, Viberti G (2010) Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 53:1838–1845
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G, ADOPT Study Group (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373:2125–2135
Dormandy J, Bhattacharya M, de Bruyn ARV, Investigators P (2009) Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes an overview of data from PROactive. Drug Saf 32:187–202
Home PD, Pocock SJ, Beck-Nielsen H et al (2005) Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD): study design and protocol. Diabetologia 48:1726–1735
Lefebvre A-M, Chen I, Desreumaux P et al (1998) Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6 J-APCmin/+ mice. Nat Med 4:1053–1057
Saez E, Tontonoz P, Nelson MC (1998) Activators of the nuclear receptor PPAR gamma enhance colon polyp formation. Nat Med 4:1058–1061
Pino MP, Kelley MF, Jayyosi Z (2004) Promotion of colon tumors in c57bl/6j-apcmin/+ mice by thiazolidinedione PPARγ agonists and a structurally unrelated PPARγ agonist. Toxicol Pathol 32:58–63
Dormandy JA, Charbonnel B, Eckland DJ et al (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macro-Vascular Events): a randomised controlled trial. Lancet 366:1279–1289
Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G (2013) Analysis cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist 18:148–156
Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Yee D et al (2010) Diabetes and cancer: a consensus report. CA Cancer J Clin 60:207–221
Hatton JL, Yee LD (2008). Clinical use of PPARγ ligands in cancer. PPAR Res. doi:10.1155/2008/159415
He X, Esteva FJ, Ensor J, Hortobagyi GN, Lee M-H, Yeung S-CJ (2012) Metformin and thiazolidinediones are associated with improved breast cancer-specific survival of diabetic women with HER2 + breast cancer. Ann Oncol 23:1771–1780
Monami M, Dicembrini I, Mannucci E (2014) Thiazolidinediones and cancer: results of a meta-analysis of randomized clinical trials. Acta Diabetol 51:91–101
Lyles BE, Akinyeke TO, Moss PE, Stewart LV (2009) Thiazolidinediones regulate expression of cell cycle proteins in human prostate cancer cells via PPARgamma-dependent and PPARgamma-independent pathways. Cell Cycle 8:268–277
Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP (2007) Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 25:1476–1481
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular disease. N Engl J Med 356:2457–2471
Acknowledgments
GlaxoSmithKline was the sponsor for the observational follow-up and the main RECORD study described in this article. We would like to thank all the patients, study coordinators, and investigators who took part in this study.
Conflict of interest
Nigel Jones and Paula Curtis are employees of GlaxoSmithKline. Philip Home or institutions with which he is affiliated receive funding from most manufacturers of glucose-lowering products for his advisory, research, or lecturing activities including GlaxoSmithKline and Takeda. All authors were involved in the design of the study, had access to the data, and were able to check its analyses. All authors took part in the writing and revision of the report.
Ethical standard
The study was approved by ethics review committees or institutional review boards in accordance with the laws and customs of each country participating in the study.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients before being included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed By Massimo Porta.
Rights and permissions
About this article
Cite this article
Jones, N.P., Curtis, P.S. & Home, P.D. Cancer and bone fractures in observational follow-up of the RECORD study. Acta Diabetol 52, 539–546 (2015). https://doi.org/10.1007/s00592-014-0691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0691-y