Abstract
Aims
Diabetic ketoacidosis (DKA) at diabetes diagnosis is a dangerous yet potentially preventable condition. Young age, low socioeconomic status, and low parental education have been found to be associated with increased risk of DKA. We aimed to evaluate the impact of religious affiliation on presentation with DKA at type 1 diabetes mellitus (T1DM) diagnosis in Jewish children.
Methods
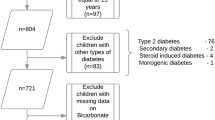
The study comprised an analysis of medical records of all consecutive patients with new-onset T1DM who were admitted to one tertiary medical center from January 2007 to January 2014. DKA was defined as venous pH <7.3 or HCO3 − < 15 mmol/l, and severe DKA as pH <7.1 or HCO3 − < 5 mmol/l.
Results
Of 81 patients with new-onset T1DM (38 females, mean ± SD age at diagnosis 9.9 ± 4.2 years), 34 (42 %) presented with DKA: 21 of 60 (35 %) of patients from secular families and 13 of 21 (62 %) from ultra-orthodox families. Children from ultra-orthodox families had a 3.5-fold increased risk of presenting with DKA than children from secular families (95 % CI 1.2–10.1, p = 0.02) and a 3.8-fold risk to be admitted with severe DKA (95 % CI 1.1–12.6, p = 0.02). Other factors that were found to be associated with an increased risk of DKA were younger age, an absence of maternal academic education, and residence in an area of low socioeconomic status.
Conclusions
DKA and severe DKA at diabetes diagnosis were more common among religious ultra-orthodox than among secular Jewish children. Awareness of the symptoms and dangers of DKA in new-onset T1DM should be directed to particularly high-risk population groups.

Similar content being viewed by others
Abbreviations
- DKA:
-
Diabetes ketoacidosis
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- MODY:
-
Maturity-onset diabetes of the young
- SES:
-
Socioeconomic status
References
Patterson CC, Dahlquist GG, Gyürüs E et al (2009) Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 373:2027–2033. doi:10.1016/S0140-6736(09)60568-7
Dabelea D, Mayer-Davis EJ, Saydah S et al (2014) Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 311:1778. doi:10.1001/jama.2014.3201
Zhao Z, Sun C, Wang C et al (2014) Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997–2011. Acta Diabetol. doi:10.1007/s00592-014-0590-2
Jarosz-Chobot P, Deja G, Polanska J (2010) Epidemiology of type 1 diabetes among Silesian children aged 0–14 years, 1989–2005. Acta Diabetol 47:29–33. doi:10.1007/s00592-009-0094-7
Usher-Smith JA, Thompson M, Ercole A, Walter FM (2012) Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia 55:2878–2894. doi:10.1007/s00125-012-2690-2
Scibilia J, Finegold D, Dorman J et al (1986) Why do children with diabetes die? Acta Endocrinol Suppl (Copenh) 279:326–333
Secrest AM, Becker DJ, Kelsey SF et al (2010) Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 59:3216–3222. doi:10.2337/db10-0862
Fernandez Castañer M, Montaña E, Camps I et al (1996) Ketoacidosis at diagnosis is predictive of lower residual beta-cell function and poor metabolic control in type 1 diabetes. Diabetes Metab 22:349–355
Mortensen HB1, Swift PG, Holl RW et al (2010) Multinational study in children and adolescents with newly diagnosed type 1 diabetes: association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes 11:218–226
Bowden SA, Duck MM, Hoffman RP (2008) Young children (<5 yr) and adolescents (>12 yr) with type 1 diabetes mellitus have low rate of partial remission: diabetic ketoacidosis is an important risk factor. Pediatr Diabetes 9:197–201. doi:10.1111/j.1399-5448.2008.00376.x
Abdul-Rasoul M, Habib H, Al-Khouly M (2006) “The honeymoon phase” in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes 7:101–107. doi:10.1111/j.1399-543X.2006.00155.x
Cameron FJ, Scratch SE, Nadebaum C et al (2014) Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 37:1554–1562. doi:10.2337/dc13-1904
Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM (2011) Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ 343:d4092
Dabelea D, Rewers A, Stafford JM et al (2014) Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 133:e938–e945. doi:10.1542/peds.2013-2795
Lévy-Marchal C, Patterson CC, Green A (2001) Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. Diabetologia 44(Suppl 3):B75–B80
De Vries L, Oren L, Lazar L et al (2013) Factors associated with diabetic ketoacidosis at onset of type 1 diabetes in children and adolescents. Diabet Med 30:1360–1366. doi:10.1111/dme.12252
Lokulo-Sodipe K, Moon RJ, Edge JA, Davies JH (2014) Identifying targets to reduce the incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in the UK. Arch Dis Child. doi:10.1136/archdischild-2013-304818
Uçar A, Saka N, Baş F et al (2013) Frequency and severity of ketoacidosis at onset of autoimmune type 1 diabetes over the past decade in children referred to a tertiary paediatric care centre: potential impact of a national programme highlighted. J Pediatr Endocrinol Metab 26:1059–1065. doi:10.1515/jpem-2013-0060
King BR, Howard NJ, Verge CF et al (2012) A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes 13:647–651. doi:10.1111/j.1399-5448.2012.00896.x
Vanelli M, Chiari G, Ghizzoni L et al (1999) Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8-year study in schools and private practices. Diabetes Care 22:7–9
Klingensmith GJ, Tamborlane WV, Wood J et al (2013) Diabetic ketoacidosis at diabetes onset: still an all too common threat in youth. J Pediatr 162:330.e1–334.e1. doi:10.1016/j.jpeds.2012.06.058
Engel-Yeger B (2012) Leisure activities preference of Israeli Jewish children from secular versus orthodox families. Scand J Occup Ther 19:341–349. doi:10.3109/11038128.2011.600330
The Central Bureau of Statistics (CBS) (2013) Israel in figures. http://www.cbs.gov.il/reader/publications/israel_fig_e.htm
Wolfsdorf J, Glaser N, Sperling MA (2006) Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American Diabetes Association. Diabetes Care 29:1150–1159. doi:10.2337/diacare.2951150
The Diabetes Control and Complications Trial (DCCT) (1986) Design and methodologic considerations for the feasibility phase. The DCCT Research Group. Diabetes 35:530–45
Dunger DB, Sperling MA, Acerini CL et al (2004) ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child 89:188–194
Blumenfeld O, Dichtiar R, Shohat T (2013) Trends in the incidence of type 1 diabetes among Jews and Arabs in Israel. Pediatr Diabetes. doi:10.1111/pedi.12101
Bui H, To T, Stein R et al (2010) Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr 156:472–477. doi:10.1016/j.jpeds.2009.10.001
Alvi NS, Davies P, Kirk JM, Shaw NJ (2001) Diabetic ketoacidosis in Asian children. Arch Dis Child 85:60–61
Sundaram PCB, Day E, Kirk JMW (2009) Delayed diagnosis in type 1 diabetes mellitus. Arch Dis Child 94:151–152. doi:10.1136/adc.2007.133405
Hilmi A, Pasternak Y, Friger M et al (2013) Ethnic differences in glycemic control and diabetic ketoacidosis rate among children with diabetes mellitus type 1 in the Negev area. Isr Med Assoc J 15:267–270
Colagiuri S (The Boden Institute of Obesity N, and Exercise, University of Sydney A) (2011) The global IDF/ISPAD guideline for diabetes in children and adolescence
Greenberg D, Witztum E (2013) Challenges and conflicts in the delivery of mental health services to ultra-orthodox Jews. Asian J Psychiatr 6:71–73. doi:10.1016/j.ajp.2012.10.008
Lightman E (Faculty of Social Work, University of Toronto, 246 Bloor Street W., Toronto, Ontario M5S1A1, Canada) Shor R (Paul Baerwald School of Social Work, Hebrew University of Jerusalem, Mt. Scopus, 91905 I) (2002) Askanim: informal helpers and cultural brokers as a bridge to secular helpers for the ultra-orthodox Jewish communities of Israel and Canada. Fam Soc 83:315–324
Rier DA, Schwartzbaum A, Heller C (2008) Methodological issues in studying an insular, traditional population: a women’s health survey among Israeli haredi (ultra-orthodox) Jews. Women Health 48:363–381. doi:10.1080/03630240802575054
Baron-Epel O, Friedman N, Lernau O (2008) Validity of self-reported mammography in a multicultural population in Israel. Prev Med (Baltim) 46:489–491. doi:10.1016/j.ypmed.2008.03.003
Katz ML, Ferketich AK, Paskett ED, Bloomfield CD (2013) Health literacy among the Amish: measuring a complex concept among a unique population. J Community Health 38:753–758. doi:10.1007/s10900-013-9675-z
Rewers A, Klingensmith G, Davis C et al (2008) Presence of diabetic ketoacidosis at diagnosis of diabetes mellitus in youth: the search for diabetes in youth study. Pediatrics 121:e1258–e1266. doi:10.1542/peds.2007-1105
Komulainen J, Lounamaa R, Knip M et al (1996) Ketoacidosis at the diagnosis of type 1 (insulin dependent) diabetes mellitus is related to poor residual beta cell function. Childhood diabetes in Finland study group. Arch Dis Child 75:410–415
Sadauskaite-Kuehne V, Samuelsson U, Jasinskiene E et al (2002) Severity at onset of childhood type 1 diabetes in countries with high and low incidence of the condition. Diabetes Res Clin Pract 55:247–254
Stein-Zamir C, Abramson N, Zentner G et al (2008) Invasive meningococcal disease in children in Jerusalem. Epidemiol Infect 136:782–789. doi:10.1017/S0950268807009259
Acknowledgments
We thank Dana Hadar, MSc, The Women and Children’s Health Research Unit, Gertner Institute, Tel Hashomer, Israel, affiliated to Sackler School of Medicine, Tel-Aviv University for a statistical assistance, and to Valentina Boyko, MSc, The Women and Children’s Health Research Unit, Gertner Institute, Tel Hashomer, Israel, affiliated to Sackler School of Medicine, Tel-Aviv University for a thorough statistical analysis. We thank Cindy Cohen, MA, for an editorial assistance.
Conflict of interest
The authors declare no conflict of interest.
Ethical standard
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Human and animal rights disclosure
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent disclosure
Due to the retrospective nature of the analysis, informed consent was not obtained, with approval of the Institutional Review Board.
Author information
Authors and Affiliations
Corresponding author
Additional information
Managed by Antonio Secchi.
Rights and permissions
About this article
Cite this article
Gruber, N., Reichman, B., Lerner-Geva, L. et al. Increased risk of severe diabetic ketoacidosis among Jewish ultra-orthodox children. Acta Diabetol 52, 365–371 (2015). https://doi.org/10.1007/s00592-014-0653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-014-0653-4