Abstract
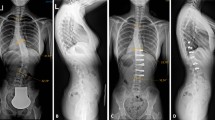
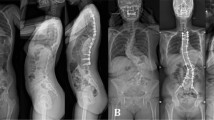
This study was conducted to refine a small animal model of scoliosis, and to quantify the deformities throughout its growth period. Subcutaneous scapula-to-contralateral pelvis tethering surgery was selected due to its minimally invasive nature and potential applicability for a large animal model. The procedure was performed in 7-week-old New Zealand white rabbits. Group A animals (n=9) underwent the tethering procedure with a suture that spontaneously released. Group B animals (n=17) had the identical procedure with a robust tether and pelvic fixation, which was maintained for 2 months during growth. All animals developed immediate post-operative scoliosis with a Cobb angle of 23° (range, 6–39°) in group A and 59° (range, 24–90°) in group B animals. During the 2 month post-tethering, group A animals lost their tether and scoliosis resolved, whereas all animals in group B maintained their tether until scheduled release at which time the mean scoliosis was 62°. Immediately after tether release, group B scoliosis decreased to a mean 53°. Over the following 4 months of adolescent growth, the scoliosis decreased to a mean of 43° at skeletal maturity; the decrease usually occurred in animals with less than 45° curves at tether release. Radiographs revealed apical vertebral wedging (mean 19°) in all group B animals. Sagittal spinal alignment was also assessed, and for group B animals, the scoliotic segment developed mild to moderate kyphosis (mean 28°) and torsional deformity, but the kyphosis resolved by 4 months after tether-release. Complications specific to this technique included a high rate of transient scapulothoracic dissociation and cases of cor pulmonale. In conclusion, this tethering technique in immature rabbits consistently produced scoliosis with vertebral wedging when the tether was intact through the first 2 months of the protocol. The transient exaggeration of kyphosis suggests that the production of scoliosis is not necessarily dependent on lordosis in this model. Because this technique does not violate thoracic or spinal tissues, it may be useful in the investigation of secondary physiologic effects of mechanically-induced scoliosis, and may be scalable to larger animal species.






Similar content being viewed by others
References
Barrios C, Arrotegui JI (1992) Experimental kyphoscoliosis induced in rats by selective brain stem damage. Int Orthop 16:146–151
Barrios C, Tunon M, De Salis J, et al (1987) Scoliosis induced by medullary damage: an experimental study in rabbits. Spine 12:433–439
Beuerlein M, Wang X, Moreau M, et al (2001) Development of scoliosis following pinealectomy in young chickens is not the result of an artifact of the surgical procedure. Microsc Res Tech 53:81–86
Binette MA, Stokes IAF, Aronsson DD, et al (1996) Mechanical modulation of intervertebral disc growth: implications for scoliosis progression. Spine 21:1162–1167
Borodic GE (1991) Chemomodulation of curvature of the juvenile spine. US Patent 5,053,005, October 1
Braun JT, Akyuz E (2005) Prediction of curve progression in a goat scoliosis model. J Spinal Disord Tech 18:272–276
Braun JT, Ogilvie JW, Akyuz E, et al (2003) Experimental scoliosis in an immature goat model: a method that creates idiopathic-type deformity with minimal violation of the spinal elements along the curve. Spine 28:2198–2203
Coillard C, Rhalmi S, Rivard CH (1999) Experimental scoliosis in the minipig: study of vertebral deformations. Ann Chir 53:773–780
Court C, Colliou OK, Chin JR, et al (2001) The effect of static in vivo bending on the murine intervertebral disc. Spine 26:239–245
Dickson RA (1987) Idiopathic scoliosis: foundation for physiological treatment. Ann R Coll Surg Engl 69:89–96
Geiger B, Steenbock H, Parsons H (1933) Lathyrism in the rat. J Nutr 6:427
Hakkarainen S (1981) Experimental scoliosis: production of structural scoliosis by immobilization of young rabbits in a scoliotic position. Acta Orthop Scand 52:1–57
Inoh H, Kawakami N, Matsuyama Y, et al (2001) Correlation between the age of pinealectomy and the development of scoliosis in chickens. Spine 26:1014–1021
Kitamura S, Ohara S, Suwa T, Nakagawa K (1965) Studies of vitamin requirements of rainbow trout, Salmo Gairdneri-I. on the ascorbic acid. J Jpn Soc Fish 31:818
Lalic JJ, Angevine DM (1970) Dysostosis in adult rats after prolonged B-aminopropyonitrile feeding. Arch Path 90:22
Lawton JO, Dickson RA (1986) The experimental basis of idiopathic scoliosis. Clin Orthop 11:9–17
Lim D, Lovel TR (1978) Pathology of the vitamin C deficiency syndrome in channel catfish (Ictalurus punetatus). J Nutr 108:1137
Machida M, Murai I, Miyashita Y, et al (1999) Pathogenesis of idiopathic scoliosis. Experimental study in rats. Spine 24:1985–1989
Masoud I, Shapiro F, Kent R, et al (1986) A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibial length. J Orthop Res 4:221–231
Mente PL, Stokes IAF, Spence H, et al (1997) Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine 22:1292–1296
Newton PO, Gollogly S, Faro F, Marks M (2004) Sagittal hypokyphosis varies with the location of the apex of the coronal curve: is there an explanation that suggests an etiology in idiopathic scoliosis. In: 39th annual meeting of the scoliosis research society, Buenos Aires, 6–9 September 2004
Pincott J, Davies JS, Taffs LF (1984) Scoliosis caused by section of dorsal spinal nerve roots. J Bone Joint Surg 66B:27–29
Robin GC (1995) Scoliosis induced by rib resection in chickens. J Spine Disord 8:179–185
Sarwark JF, Dabney KW, Salzman SK, et al (1988) Experimental scoliosis in the rat. I. Methodology, anatomic features, and neurologic characterization. Spine 13:466–471
Smith R, Dickson RA (1987) Experimental structural scoliosis. J Bone Joint Surg 69B:576–581
Stokes IA, Aronsson DD (2001) Disc and vertebral wedging in patients with progressive scoliosis. J Spinal Disord 14:317–322
Stokes IA, Mente PL, Iatridis JC, et al (2002) Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg 84A:1842–1848
Thomas S, Dave PK (1985) Experimental scoliosis in monkeys. Acta Orthop Scand 56:43–46
Thometz JG, Liu XC, Lyon R (2000) Three-dimensional rotations of the thoracic spine after distraction with and without rib resection: a kinematic evaluation of the apical vertebra in rabbits with induced scoliosis. J Spinal Disord 13:108–112
Tsuang YH, Yang RS, Chen PQ, et al (1992) Experimental structural scoliosis in rabbits. J Formos Med Assoc 91:886–890
Wang X, Moreau M, Raso J, et al (1998) Changes in serum melatonin levels in response to pinealectomy in the chicken and its correlation with development of scoliosis. Spine 23:2377–2381
Whittle MW, Evans ML (1979) Instrument for measuring the Cobb angle in scoliosis. Lancet 1:414
Acknowledgement
The authors thank Inion, Ltd. for providing the financial support required to complete this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kallemeier, P.M., Buttermann, G.R., Beaubien, B.P. et al. Validation, reliability, and complications of a tethering scoliosis model in the rabbit. Eur Spine J 15, 449–456 (2006). https://doi.org/10.1007/s00586-005-1032-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-005-1032-1