Abstract
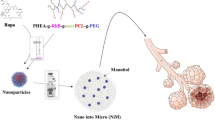
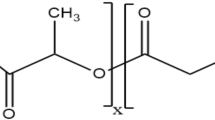
Poly lactic-co-glycolic acid (PLGA), a biodegradable polymer, can effectively protect encapsulated peptides from enzymatic degradation. PLGA was approved by FDA as a safe drug delivery system suitable for inhalation administration. Vasoactive intestinal peptide (VIP), a 28-amino-acid peptide, displays anti-inflammatory and anti-spasmodic effects, which can be considered as a new therapeutic option to control and treat asthma. Because of in vivo enzymatic degradation of VIP including in the lung, there is a need for an applicable delivery system. In light of this, the purpose of this study was to prepare VIP-loaded PLGA microspheres as a drug delivery system, assuming that the newly-introduced model has the ability to persist for a longer time in respiratory tracts. The PLGA microsphere was produced, and loaded with VIP as an applicable nanodrug system. A series of physiochemical properties were determined, including the morphological characteristics, average size of nanoparticles, electric charge distribution, FTIR spectroscopy absorption, and loading and releasing percentage of the nanodrug. VIP-loaded PLGA exhibited an average size of approximately 550 ± 50 nm. Additionally, the produced microsphere showed 78 % VIP release after 10 h at the pH value corresponding to bronchioalveolar microenvironment (approximately 6.5). In the present study, PLGA was formulated and used as a delivery system for VIP. Taken together, the newly-introduced nanodrug seems to be helpful for the clinical treatment of allergic asthma. PLGA nanoparticles can be considered as a potential efficient delivery system for VIP in the respiratory system.




Similar content being viewed by others
References
Amaral AC, Boca AL, Ribeiro AM, Nunes J (2009) Amphotericin B in poly(lactic-coglycolic acid) (PLGA) and dimercaptosuccinic acid (DMSA) nanoparticles against paracoccidioidomycosis. J Antimicrob Chemother 63:529–33
Bivas-Benita M, Romeijn S, Junginger HE, Borchard G (2004) PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur J Pharm Biopharm 58:1–6
Campolongo MJ, Luo D (2009) Drug delivery: old polymer learns new tracts. Nat Mater 8(6):447–448
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–522
Emami J, Hamishehkar H, Najafabadi AR, Gilani K, Minaiyan M, Mahdavi H, Mirzadeh H, Fakhari A, Nokhodchi A (2009) Particle size design of PLGA microspheres for potential pulmonary drug delivery using response surface methodology. J Microencapsul 26(1):1–8
Erbetta CD’AC, Alves RJ, Resende JM, de Souza Freitas RF, de Sousa RG (2012) Synthesis and characterization of poly (D, L-Lactide-co-Glycolide) copolymer. J Biomater Nanobiotechnol 3:208–225
Faisant N, Akiki J, Siepmann F, Benoit JP, Siepmann J (2006) Effects of the type of release medium on drug release from PLGA-based microparticles: experiment and theory. Int J Pharm 314:189–197
Heng D, Cutler DJ, Chan HK, Yun J, Raper JA (2008) What is a suitable dissolution method for drug nanoparticles? Pharm Res 25:1696–701
Igarashi H, Ito T, Mantey SA et al (2005) Development of simplified vasoactive intestinal peptide analogs with receptor selectivity and stability for human vasoactive intestinal peptide/pituitary adenylate cyclase-activating polypeptide receptors. J Pharmacol Exp Ther 315(1):370–381
Jain RA (2000) The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide) (PLGA) devices. Biomaterials 21:2475–90
Jensen DMK, Cun D, Maltesen MJ, Frokjaer S, Nielsen HM, Foged C (2010) Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J Control Release 142:138–145
Lee W-H, Loo C-Y, Traini D, Young PM (2015) Inhalation of nanoparticle-based drug for lung cancer treatment: advantages and challenges. Asian J Pharma Sci 10(6):481–489
Linden A, Hansson L, Andersson A et al (2003) Bronchodilation by an inhaled VPAC2 receptor agonist in patients with stable asthma. Thorax 58(3):217–221
Lü J-M, Wang X, Marin-Muller C, Wang H, Lin PH, Qizhi Y, Changyi C (2009) Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn 9(4):325–341
Misaka S, Aoki Y, Karaki S-i, Kuwahara A, Mizumoto T, Onoue S, Yamada S (2010) Inhalable powder formulation of a stabilized vasoactive intestinal peptide (VIP) derivative: anti-inflammatory effect in experimental asthmatic rats. Peptides 31:72–78
Moody TW, Nuche-Berenguer B, Jensen RT (2016) Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr Opin Endocrinol Diabetes Obes 23(1):38–47
Mundargi RC, Ramesh Babu V, Rangaswamy V, Patel P, Aminabhavi TM (2008) Nano/micro technologies for delivering macromolecular therapeutics using poly(D, L-lactide-co-glycolide) and its derivatives. J Control Release 125(3):193–209
Onoue S, Matsui T, Kuriyama K, Ogawa K, Kojo Y, Mizumoto T, Karaki S-i, Kuwahara A, Yamada S (2012) Inhalable sustained-release formulation of long-acting vasoactive intestinal peptide derivative alleviates acute airway inflammation. Peptides 35:182–189
Pirooznia N, Hasannia S, Lotfi AS, Ghanei M (2012) Encapsulation of alpha-1 antitrypsin in PLGA nanoparticles: in vitro characterization as an effective aerosol formulation in pulmonary diseases. J Nanobiotechnol 10:20
Pohlmann AR, Weiss V, Mertins O (2002) Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres development, stability evaluation and nanostructure models. Eur Pharm Sci 16:305–312
Prasse A, Zissel G, Lutzen N et al (2010) Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med 182(4):540–548
Semete B, Booysen L, Lemmer Y, Kalombo L, Katata L, Verschoor J, Swai HS (2010) In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomed: Nanotechnol, Biol Med 6:662–671
Takashima Y, Saito R, Nakajima A, Oda M, Kimura A, Kanazawa T, Okada H (2007) Spray-drying preparation of microparticles containing cationic PLGA nanospheres as gene carriers for avoiding aggregation of nanospheres. Int J Pharm 343:262–269
Tseng C-L, Wu SY-H, Wang W-H, Peng C-L, Lin F-H, Lin C-C, Young T-H, Shieh M-J (2008) Targeting efficiency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer. Biomaterials 29(20):3014–3022
Ungaro F, d’Angelo I, Ciro C, di Villa Bianca R d’E, Raffaella S, Brunella P, Maria Antonietta T, Agnese M, Maria Immacolata La R, Fabiana Q (2012) Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Control Release 157:149–159
van Noort JM, Bsibsi M, Nacken PJ, Gerritsen WH, Amor S, Holtman IR, Boddeke E, van Ark I, Leusink-Muis T, Folkerts G, Hennink WE, Amidi M (2013) Activation of an immune-regulatory macrophage response and inhibition of lung inflammation in a mouse model of COPD using heat-shock protein alpha B-crystallin-loaded PLGA microparticles. Biomaterials 34:831–840
Vicari L, Musumeci T, Giannone I, Adamo L, Conticello C, De Maria R, Pignatello R, Puglisi G, Gulisano M (2008) Paclitaxel loading in PLGA nanospheres affected the in vitro drug cell accumulation and antiproliferative activity. BMC Cancer 8:212
Yamamoto H, Kuno Y, Sugimoto S, Takeuchi H, Kawashima Y (2005) Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J Control Release 102(2):373–381
Zhang N, Chittapuso C, Ampassavate C, Siahaan TJ, Berkland C (2008) PLGA nanoparticle-peptide conjugate effectively targets intercellular cell-adhesion molecule-1. Bioconjug Chem 19(1):145–152
Zolnik BS, Burgess DJ (2007) Effect of acidic pH on PLGA microsphere degradation and release. J Control Release 122:338–344
Acknowledgments
This manuscript was based on a research work performed as a partial fulfillment for PhD degree in medical immunology that was supported by Tarbiat Modares University. The authors also acknowledge Dr. Mehdi Shafiee Ardestani; the Immunology, Asthma and Allergy Research Institute, Children’s Medical Center, Tehran University of Medical Sciences, Tehran, Iran; Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran; Utrecht Institute for Pharmaceutical Sciences, Faculty of Sciences, Utrecht University, Utrecht, Netherlands and Airways Disease Section, National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors. This study has ethical approval from the Asthma and Allergy Research Institute, Children’s Medical Center, Tehran University of Medical Sciences with No. 412/P/236 and from the Faculty of Medical Sciences, Tarbiat Modares University with No. 52D/6564.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Financial support
This study was funded by Tarbiat Modares University (52D/313), Utrecht Institute for Pharmaceutical Sciences, Faculty of Sciences, Utrecht University and Immunology, Asthma and Allergy Research Institute, Children’s Medical Center, Tehran University of Medical Sciences (412/P/135).
Rights and permissions
About this article
Cite this article
Athari, S.S., Mortaz, E., Pourpak, Z. et al. VIP-loaded PLGA as an anti-asthma nanodrug candidate. Comp Clin Pathol 25, 791–796 (2016). https://doi.org/10.1007/s00580-016-2265-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-016-2265-6