Abstract
Porcine reproductive and respiratory syndrome (PRRS) virus infection often lead to infertility in gilts and sows. Nevertheless, the impact of PRRS virus on the endometrial function has not been fully elucidated. The present study aimed to determine the effect of PRRS virus infection on the expression of oestrogen receptor (ER) α in the endometrium of gilts. Uterine tissues from 54 gilts were classified into two groups according to PRRS virus detection using immunohistochemistry (26 positive and 28 negative samples). The reproductive status was classified as prepubertal, luteal and follicular phases. The expression of ERα in the epithelium, subepithelium and glandular tissue layers of the endometrium was determined by immunohistochemistry. ERα immunostaining was detected in 22.2, 13.5 and 33.6 % of the cells in the epithelial, subepithelial and glandular tissue layers, respectively. The ERα immunostaining in the glandular layer of the endometrium in follicular-phase gilts was higher than that in prepubertal gilts (46.1 and 15.2 %, respectively, P = 0.011). The ERα immunostaining in the glandular layer of the endometrium was positively correlated with body weight (r = 0.138, P = 0.020) and average daily gain (r = 0.169, P = 0.005) of the gilts. The ERα immunostaining in all tissue layers of the endometrium with PRRS virus detection did not differ significantly compared to that without PRRS virus (P > 0.05). However, in prepubertal gilts, the ERα immunostaining cells in the epithelial layer of PRRS virus negative (9.5 %) tended to be lower than PRRS virus positive (26.3 %, P = 0.196) uterine tissues. This tendency indicates some impact of PRRS virus infection on the ERα expression in prepubertal gilts.
Similar content being viewed by others
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is an economically important disease in the pig industry worldwide. In general, reproductive failures caused by PRRS virus is characterised by late-term abortion, mummified foetuses, stillborn piglets, a decreased number of piglets born alive per litter and an increased number of low vitality newborn piglets (Olanratmanee et al. 2013). In general, the economic impact due to PRRS infection in commercial swine-herds includes an increase in non-productive days, an increase in the replacement rate and a decreased number of piglets weaned per sow per year. PRRS is caused by the PRRS virus, which is a single-stranded RNA virus belonging to family Arteriviridae and order Nidovirales (Jantafong et al. 2015). The PRRS virus is widespread among swine populations and affects pigs of all ages (Sur et al. 2001; Olanratmanee et al. 2013). In pregnant sows, PRRS virus infection can occur at any stage of gestation. However, in most cases, the exposure of sows to the PRRS virus causes reproductive failure during late gestation (Mengeling et al. 1998; Karniychuk et al. 2011). In our previous study, the PRRS virus was detected in 33 % of the uterine tissues in gilts culled due to reproductive disturbance (Olanratmanee et al. 2011). PRRS virus target cells, i.e., mainly macrophages, are commonly seen in the endometrium of the gilts (Teamsuwan et al. 2010). Surprisingly, the detection frequency of PRRS virus in the uterine tissue did not differ significantly between non-mated gilts and mated gilts (Olanratmanee et al. 2011). Furthermore, the PRRS virus remains in the uterine tissue of the infected gilts for several months even though vaccinations and/or acclimatisation protocols are regularly implemented (Olanratmanee et al. 2011). It is hypothesised that the persistence of PRRS virus in the uterine tissue of gilts may compromise the uterine function and cause infertility in gilts. Karniychuk et al. (2011) showed that the PRRS virus can replicate and induce apoptosis in the foetal implantation sites during late gestation, which indicates that PRRS virus infection may cause pathological changes in the porcine endometrium and subsequently lead to infertility. The effect of PRRS virus infection on the endometrial function in gilts and sows is still poorly understood, and the relationship between PRRS virus and endometrial function remains to be explored.
Oestrogens play important roles in regulating the activities of the porcine uterus (Sukjumlong et al. 2003). The most active form of oestrogen, oestradiol-17β, is synthesised by follicles. Theca interna and granulosa cells are the main component of the follicles that synthesise oestrogen (Watanabe et al. 1997). The function of oestrogens in the porcine uterus is mediated by oestrogen receptor (ER). In general, two isoforms of ER have been described, i.e., ERα and ERβ. ERα is the isoform that predominantly regulates reproductive behaviour and physiology in females, and it is an important regulator of the reproductive function through ligand-dependent and -independent mechanisms in the porcine uterus (Sukjumlong et al. 2003). ERα is distributed within all compartments of the porcine uterine tissue, i.e., surface epithelium, subepithelium, glandular epithelium and myometrium (Sukjumlong et al. 2004). However, the expression of ERα varies depending on many events that occur in the uterus, e.g., stage of the oestrus cycle, artificial insemination, pregnancy and bacterial infection (Srisuwatanasagul 2011). Thus, ERα expression is strongly associated with the porcine uterine function. Nevertheless, the relationship between PRRS virus in the porcine uterus (Olanratmanee et al. 2011) and endometrial function has not been investigated. Thus, the objective of the present study was to determine the effect of the presence of PRRS virus on the expression of ERα in the endometrium of gilts.
Materials and methods
Tissue samples, data and experimental design
Uterine tissues from 54 Landrace × Yorkshire crossbred gilts were obtained from our previous study (Tummaruk et al. 2009). The presence of PRRS virus in the uterine tissue of the gilts was determined in our previous study using immunohistochemistry (Olanratmanee et al. 2011). The uterine tissues were classified into two groups according to the presence of PRRS virus, i.e., PRRS virus positive (n = 26) and PRRS virus negative (n = 28) uterine tissues. The immunostaining of ERα in all tissue layers of the endometrium was carefully determined. The historical background data of each sample were also collected. Reproductive organs of the culled gilts together with their historical data were obtained from slaughter-houses. The data included the herd and gilt identity, breed, date of birth, date of entry into the herd, date of culling, body weight at culling and reason for culling. The age at culling (days) and average daily gain (g/day) was calculated (Tummaruk et al. 2009). The organs were collected immediately after slaughter and were placed on ice and transported to the laboratory within 24 h. The ovarian structures, including the corpora lutea (CL), corpora albicantia (CA) and follicles were defined according to a previous study (Tummaruk et al. 2009). The appearance of the ovaries was used to define the reproductive status. The reproductive status was defined as prepubertal when the ovaries contained only small follicles and had no CL or CA. The luteal phase was defined as when the ovaries contained CL or CA, and the follicular phase was defined as when the ovary contained dominant follicles (Tummaruk et al. 2015). The uterine tissues were classified according to their ovarian status, i.e., prepubertal (n = 12), luteal phase (n = 26) and follicular phase (n = 16).
Tissue processing and immunohistochemistry
The uterine tissues were fixed in 10 % neutral-buffered formalin for 24–48 h, processed by an automatic tissue processor (Tissue-Tek VIP 5 Jr., Sakura, Tokyo, Japan) and embedded in a paraffin block (Tissue-Tek TEC, Sakura). Thereafter, the paraffin blocks were cut into 4.0-μm-thick sections using a microtome (Shandon, Anglia Scientific Instruments Ltd, Cambridge, UK). The uterine tissues were deparaffinized in xylene and rehydrated through graded ethanol dilutions. The tissue sections were placed in 0.01 M citrate buffer (pH 6.0) in a microwave oven at 750 W for 15 min (3 × 5 min) to retrieve antigenicity. Endogenous peroxidase activity was inhibited by immersing the tissue sections in freshly prepared 3.0 % hydrogen peroxide at room temperature for 10 min. Non-specific staining was blocked with normal horse serum at room temperature for 30 min. The slides were incubated with a mouse monoclonal antibody against ERα (clone MIB-1, Dako Denmark A/S, Glostrup, Denmark) as a primary antibody at a dilution of 1:50 at room temperature for 120 min. After incubation with the primary antibody, the sections were incubated with secondary antibody, biotinylated-horse anti-mouse anti-rabbit immunoglobulin G (1:200, Vector Laboratories, Burlingame, CA, USA), for 30 min at room temperature and an avidin-biotin-peroxidase complex (30 min at room temperature, Elite, Vectastain®, ABC kit, Vector Laboratories). Staining was developed with 3,3′-diaminobenzidine tetrahydrochloride hydrate (2 min at room temperature, Peroxidase DAB, Vector Laboratories). The sections were counterstained with Mayer’s haematoxylin for 1 min, and mounted with mounting media. During staining, the slides were washed three times in PBS for 5 min each. For the negative control, PBS was used instead of the primary antibody.
ERα immunostaining
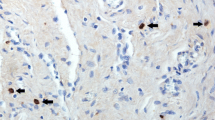
ERα immunostaining was determined in epithelial, subepithelial and glandular tissue layers of the endometrium. The ERα-positive immunostained cells were evaluated using Image-Pro® Plus software (Media Cybernetics, Inc., MD, USA) under a light microscope (Fig. 1). Five objective fields from each uterine section were randomly selected for immunohistochemical evaluation. In total, 270 microscopic fields (54 tissues × 5 fields) in each of the epithelial, subepithelial and glandular tissue layers of the endometrium were evaluated. The percentage of ERα-positive cells was calculated: ERα-positive cells (%) = [(number of ERα-positive cells / total number of cells counted) × 100].
Expression of oestrogen receptor (ER) α in the endometrium of gilts. a Negative control, b epithelial layer, c subepithelial layer and d glandular tissue layer of the endometrium with ERα immunostaining. Black arrows indicate negative ERα immunostaining cells and black arrowheads indicate positive ERα immunostaining cells (×200 magnification, bar 20 μm)
Statistical analysis
The statistical analyses were carried out using SAS version 9.0 (SAS institute Inc., Cary, NC, USA). Descriptive statistics, including general means, standard deviation (SD) and the range of all the continuous data, were calculated. Pearson’s correlation was used to determine the correlation between ERα expression in each tissue layer of the endometrium and age, average daily gain and body weight of the gilts. Multiple analysis of variance (ANOVA) was used to analyse the ERα-positive cells in each tissue layer of the endometrium using the general linear mixed model procedure of SAS. Dependent variables included the ERα-positive immunostained cells in the epithelial, subepithelial and glandular tissue layers of the endometrium. The statistical models included the effect of reproductive status (prepubertal, luteal and follicular phases), PRRS virus detection (positive and negative), and the interaction between reproductive status and the presence of PRRS virus. The gilt’s identity, nested within reproductive status and PRRS virus detection, was included in the models as a random effect. Least-squares means ± standard error of the mean (SEM) were calculated and were compared between classes of the independent variables by using the least significant difference test. P < 0.05 was regarded as statistically significant.
Results
On average, the age, body weight and average daily gain of the gilts were 299 ± 50 days, 150 ± 22 kg and 502 ± 68 g/day, respectively. Age, body weight and average daily gain of the gilts with and without PRRS virus detection in the uterine tissues did not differ significantly (P > 0.05; Table 1). Immunostaining of ERα in the uterine tissues of gilts was present in all layers of the endometrium (Fig. 1). ERα immunostaining was detected in 22.2, 13.5 and 33.6 % of the cells in the epithelial, subepithelial and glandular tissue layers, respectively. The ERα immunostaining in the glandular layer of the endometrium was positively correlated with body weight (r = 0.138, P = 0.020) and average daily gain (r = 0.169, P = 0.005) of the gilts (Table 2). Gilts with a high average daily gain had high ERα immunostaining in the glandular epithelial layer of the endometrium (P = 0.005).
Regardless of the reproductive status, the ERα immunostaining in all tissue layers of the endometrium did not differ significantly between the uterine tissue with and without the presence of PRRS virus (Table 3). However, the ERα immunostaining in the glandular layers of the endometrium differed between reproductive statuses (P < 0.05; Table 4). The ERα immunostaining in the glandular layers of the endometrium in follicular-phase gilts was higher than that in prepubertal gilts (46.1 and 15.2 %, respectively, P = 0.011).
The ERα expression in all tissue layers of the endometrium in the uterine tissue with PRRS virus and without the PRRS virus by reproductive status is shown in Table 5. No significant difference in the ERα expression was found between the uterine tissues with and without the presence of PRRS virus (P > 0.05). However, in prepubertal gilts, the ERα immunostaining cells in the epithelial layer of PRRS virus negative (9.5 %) tended to be lower than PRRS virus positive (26.3 %, P = 0.196) uterine tissues (Table 5).
Discussion
The present study demonstrated that the percentage of ERα immunostaining did not differ significantly between the uterine tissues with and without PRRS virus detection. This indicates that the PRRS virus did not have a major impact on the expression of ERα in the non-pregnant porcine endometrium. To our knowledge, this finding has never been reported before. In pregnant animals, Karniychuk et al. (2011) demonstrated that PRRS virus target cells are presented in the endometrium of sows and also in the foetal placenta. PRRS virus infection increases apoptosis of macrophages and surrounding cells in the porcine placenta, especially during late gestation (Karniychuk et al. 2011). The maternal viraemia may lead to PRRS virus replication in the endometrium with subsequent foetus infection through the foetal placenta. However, the severity of PRRS virus infection on the uterine function in gilts or sows depends on the stage of gestation. Based on these findings, the PRRS virus affects the uterine function during the late stage of gestation rather than at early stages of gestation. Nevertheless, the impact of PRRS virus infection on the uterine function of non-pregnant gilts and sows is still poorly understood. Olanratmanee et al. (2011) detected the PRRS virus in formalin-fixed and paraffin-embedded uterine tissues in 29.6 to 40.9 % of breeding-age replacement gilts (i.e., 6–16 months of age), which indicates the risk of infertility due to PRRS virus infection in replacement gilts. Under field conditions, return to oestrus after mating was found in 16.3 % of gilts compared to 6.7 % of multiparous sows (Tummaruk et al. 2010b). Furthermore, Tummaruk et al. (2009) demonstrated that commons reasons for culling of replacement gilts included anoestrus, abnormal vaginal discharge, repeat breeding and not being pregnant. Additionally, Tummaruk and Tantilertcharoen (2012) found that at least 73 % of the gilts that were culled due to reproductive failure in Thailand had been infected with the PRRS virus. Recently, Tummaruk et al. (2015) found PRRS virus in 70 % of the ovarian tissues from gilts culled due to reproductive failure. These data indicate that poor fertility of replacement gilts in relation to some infectious causes, e.g., PRRS virus, should be emphasised. The PRRS virus has been one of the most common infectious causes of infertility in swine breeding herds worldwide over the past 20 years. However, knowledge on the mechanism of PRRS virus infection on uterine function is limited.
In the present study, the ERα immunostaining in the epithelial layer of PRRS virus negative prepubertal phase was almost three times lower than that in the same layer of PRRS virus positive prepubertal phase. Although, the difference was not statistically significant (P = 0.196), an interesting tendency was observed. The reason is not known, but this tendency might indicate some impact of PRRS virus infection on the ERα expression in prepubertal gilts. Therefore, further research needs to focus on the influence of PRRS infection in prepubertal gilts. In addition, the influence of PRRS virus infection on the uterine function of gilts via other mechanisms is also needed to be explored.
It is well established that bacterial infection in the uterus caused various degree of uterine dysfunction. Its impact can be varied from mild to severe. Endometritis is one of the most common uterine disease in pigs (Tummaruk et al. 2009). Based on the clinical symptoms (i.e., the presence of pus exudate) and bacterial culture, bacteria (e.g., E. coli, Streptococcus sp.) were suspected to be the primary cause (Tummaruk et al. 2010a). However, recent studies on the endometrial function in cow indicated that co-infection between bovine herpes virus 4 and E. coli is closely associated with the severity of the clinical signs of postpartum endometritis (Donofrio et al. 2008; Chastant-Maillard 2015). In pigs, this aspect has never been done. Since the presence of PRRS virus in the endometrium of gilts with reproductive disorders has been demonstrated (Olanratmanee et al. 2011), research on its influences on the uterine function is therefore of interest.
In normal gilts, the expression of ERα is high during oestrus to enhance the endometrial and myometrial activities (Sukjumlong et al. 2004). The changes in the ERα expression in the endometrium may alter the uterine contraction and reduce the ability of the uterus to eliminate abnormal secretion and/or bacterial infection, which may also subsequently cause an impaired uterine function (de Ziegler et al. 2001). Srisuwatanasagul et al. (2010) found that ERα immunostaining was found mainly in the uterine stroma and the myometrium. This indicates that the uterine stromal and myometrial cells respond to the changes of the circulating sex hormones and cause an alteration in the hormone receptors. In the present study, the ERα expression was detected in all tissue layers of the endometrium, which indicates that the uterine tissues of the gilts at this age (i.e., 299 days) are ready for stimulation by ovarian steroid hormones. In Thailand, gilts normally attain puberty at about 200 days of age (Tummaruk 2012). Therefore, the expression of ERα in the uterine tissues of the gilts in the present study is not surprising, although some variation in the ERα expression was observed. Interestingly, the expression of ERα in the endometrium of gilts was positively correlated with both the average daily gain and the body weight of the gilts. This implies that, at the same age, gilts with better growth performance and body weight also had a higher ERα expression compared to those with poorer growth. Thus, selection of replacement gilts with superior growth and/or body weight might improve their reproductive function. This is in accordance with the previous study that showed that gilts with a better average daily gain and higher body weight were mated earlier and had a larger litter size than those with a poorer average daily gain and lower body weight (Roongsitthichai et al. 2013).
Regarding the influence of reproductive status on the ERα immunostaining in the epithelia and glandular tissue layers of the endometrium, follicular-phase gilts had a higher ERα expression than prepubertal gilts. To our knowledge, the comparison of ERα immunostaining between prepubertal gilts and cyclic gilts at the same age has never been done. The high ERα immunostaining in the endometrium in follicular-phase gilts might be due to the fact that the level of ovarian steroid hormones in the follicular-phase gilts influences the expression of ERα (Srisuwatanasagul et al. 2010).
In conclusion, the ERα expression in all tissue layers of the endometrium containing PRRS virus did not differ significantly compared to the endometrium without PRRS virus. This indicates that PRRS virus infection did not alter the immunostaining of ERα in the porcine endometrium. However, reproductive status, average daily gain and body weight of the gilts significantly influenced the ERα expression in the porcine endometrium. High ERα expression was observed in follicular-phase gilts and in gilts with a high average daily gain and/or high body weight.
References
Chastant-Maillard S (2015) Impact of bovine herpesvirus 4 (BoHV-4) on reproduction. Transbound Emerg Dis 62:245–251
de Ziegler D, Bulletti C, Fanchin R, Epiney M, Brioschi PA (2001) Contractility of the nonpregnant uterus: the follicular phase. Ann N Y Acad Sci 943:172–184
Donofrio G, Ravanetti L, Cavirani S, Herath S, Capocefalo A, Sheldon LM (2008) Bacterial infection of endometrial stroma cells influences bovine herpesvirus 4 immediate early gene activation: a new insight into bacterial and viral interaction for uterine disease. Reproduction 136:361–366
Jantafong T, Sangtong P, Saenglub W, Mungkundar C, Romlamduan N, Lekchareonsuk C, Lekcharoensuk P (2015) Genetic diversity of porcine reproductive and respiratory syndrome virus in Thailand and Southeast Asia from 2008 to 2013. Vet Microbiol 176:229–238
Karniychuk UU, Saha D, Geldhof M, Vanhee M, Cornillie P, van den Breock W, Nauwynck HJ (2011) Porcine reproductive and respiratory syndrome virus (PRRSV) causes apoptosis during its replication in fetal implantation sites. Microb Pathog 51:194–202
Mengeling WL, Lager KM, Vorvald CA (1998) Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am J Vet Res 59:1540–1544
Olanratmanee E, Wangnaitham S, Thanawongnuwech R, Kunavongkrit A, Tummaruk P (2011) Prevalence of porcine reproductive and respiratory syndrome virus (PRRSV) antigen-positive uterine tissues in gilts culled due to reproductive disturbance in Thailand. Trop Anim Health Prod 43:451–457
Olanratmanee E, Nuntawan Na Ayudhya S, Thanawongnuwech R, Kunavongkrit A, Tummaruk P (2013) Reproductive parameters following a PRRS outbreak where a whole-herd PRRS MLV vaccination strategy was instituted post-outbreak. Trop Anim Health Prod 45:1099–1106
Roongsitthichai A, Cheuchuchart P, Chatwijitkul S, Chantarothai O, Tummaruk P (2013) Influence of age at first estrus, body weight, and average daily gain of replacement gilts on their subsequent reproductive performance as sows. Livest Sci 151:238–245
Srisuwatanasagul S (2011) Steroid receptor and their roles in pig uterus. Thai J Vet Med 41(supplement 1):43–49
Srisuwatanasagul S, Tummaruk P, Kunavongkrit A (2010) Studies of oestrogen and progesterone receptors in reproductive organs of prepubertal gilts with reproductive disturbance. Thai J Vet Med 40:15–24
Sukjumlong S, Kaoeket K, Dalin A-M, Persson E (2003) Immunohistochemical studies on oestrogen receptor alpha (ER∞) and the proliferative marker Ki-67 in the sow uterus at different stages of the oestrus cycle. Reprod Domest Anim 38:5–12
Sukjumlong S, Persson E, Kaeoket K, Dalin A-M (2004) Immunohistochemical studies on oestrogen receptor alpha (ER∞) and the proliferative marker Ki-67 in the sow uterus at oestrus and early pregnancy. Reprod Domest Anim 39:361–369
Sur JH, Doster AR, Galeota JA, Osorio FA (2001) Evidence for the localization of porcine reproductive and respiratory syndrome virus (PRRSV) antigen and RNA in ovarian follicles in gilts. Vet Pathol 38:58–66
Teamsuwan Y, Kaeoket K, Tienthai P, Tummaruk P (2010) Morphological changes and infiltration of immune cells in the endometrium of anoestrus gilt in relation to the ovarian appearance and serum progesterone. Thai J Vet Med 40:31–40
Tummaruk P (2012) Effects of season, outdoor climate and photo period on age at first observed estrus in Landrace X Yorkshire crossbred gilts in Thailand. Livest Sci 144:163–172
Tummaruk P, Tantilertcharoen R (2012) Seroprevalence of porcine reproductive and respiratory syndrome, Aujeszky’s disease and porcine parvovirus in replacement gilts in Thailand. Trop Anim Health Prod 44:983–989
Tummaruk P, Kesdangsakonwut S, Kunavongkrit A (2009) Relationships among specific reason for culling, reproductive data and gross-morphology of the genital tracts in gilts culled due to reproductive failure in Thailand. Theriogenology 71:369–375
Tummaruk P, Kesdangsakonwut S, Prapasarakul N, Kaeoket K (2010a) Endometritis in gilts: reproductive data, bacterial culture, histopathology, and infiltration of immune cells in the endometrium. Comp Clin Pathol 19:575–584
Tummaruk P, Tantasuparuk W, Techakumphu M, Kunavongkrit A (2010b) Influence of repeat-service and weaning-to-first-service interval on farrowing proportion of gilts and sows. Prev Vet Med 96:194–200
Tummaruk P, Phoophitphong D, Olanratmanee E, Thanawongnuwech R (2015) Detection of porcine reproductive and respiratory syndrome virus in the ovary of gilts culled due to reproductive disturbances. Comp Clin Pathol 24:903–910
Watanabe T, Inoue S, Ogawa S, Ishii Y, Hiroi H, Ikeda K, Orimo A, Muramatsu M (1997) Agonistic effect of tamoxifen is dependent on cell type, pre-promoter context, and estrogen receptor subtype: functional difference between estrogen receptors α and β. Biochem Biophys Res Commun 236:140–145
Acknowledgements
Financial support for the study was provided by a Grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund. D. Phoophitphong is a grantee of the Royal Golden Jubilee (RGJ) Ph.D. Program, the Thailand Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tummaruk, P., Boonwong, N., Chumthong, W. et al. Expression of oestrogen receptor α in the endometrium of porcine reproductive and respiratory syndrome virus-infected gilts. Comp Clin Pathol 25, 549–554 (2016). https://doi.org/10.1007/s00580-016-2226-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-016-2226-0