Abstract
Purpose
Propofol inhibits the amplitudes of transcranial electrical motor-evoked potentials (TCE-MEP) in a dose-dependent manner. However, the mechanisms of this effect remain unknown. Hence, we investigated the spinal mechanisms of the inhibitory effect of propofol on TCE-MEP amplitudes by evaluating evoked electromyograms (H-reflex and F-wave) under general anesthesia.
Methods
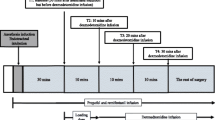
We conducted a prospective, single-arm, interventional study including 15 patients scheduled for spine surgery under general anesthesia. Evoked electromyograms of the soleus muscle and TCE-MEPs were measured at three propofol concentrations using target-controlled infusion (TCI: 2.0, 3.0, and 4.0 µg/mL). The primary outcome measure was the left H-reflex amplitude during TCI of 4.0- compared to 2.0-µg/mL propofol administration.
Results
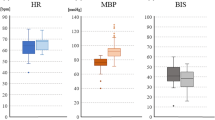
The median [interquartile range] amplitudes of the left H-reflex were 4.71 [3.42–6.60] and 5.6 [4.17–7.46] in the 4.0- and 2.0-μg/mL TCI groups (p = 0.4, Friedman test), respectively. There were no significant differences in the amplitudes of the right H-reflex and the bilateral F-wave among these groups. However, the TCE-MEP amplitudes significantly decreased with increased propofol concentrations (p < 0.001, Friedman test).
Conclusion
Propofol did not affect the amplitudes of the H-reflex and the F-wave, whereas TCE-MEP amplitudes were reduced at higher propofol concentrations. These results suggested that propofol can suppress the TCE-MEP amplitude by inhibiting the supraspinal motor pathways more strongly than the excitability of the motor neurons in the spinal cord.


Similar content being viewed by others
References
Pelosi L, Lamb J, Grevitt M, Mehdian S, Webb J, Blumhardt L. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113:1082–91.
Langeloo DD, Lelivelt A, Journée HL, Slappendel R, de Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa 1976). 2003;28:1043–50.
Gonzalez AA, Jeyanandarajan D, Hansen C, Zada G, Hsieh PC. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27:E6.
Park P, Wang AC, Sangala JR, Kim SM, Hervey-Jumper S, Than KD, Farokhrani A, LaMarca F. Impact of multimodal intraoperative monitoring during correction of symptomatic cervical or cervicothoracic kyphosis. J Neurosurg Spine. 2011;14:99–105.
Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19:430–43.
Haghighi SS, Green KD, Oro JJ, Drake RK, Kracke GR. Depressive effect of isoflurane anesthesia on motor evoked potentials. Neurosurgery. 1990;26:993–7.
Kalkman CJ, Drummond JC, Ribberink AA, Patel PM, Sano T, Bickford RG. Effects of propofol, etomidate, midazolam, and fentanyl on motor evoked responses to transcranial electrical or magnetic stimulation in humans. Anesthesiology. 1992;76:502–9.
Haghighi SS, Sirintrapun SJ, Keller BP, Oro JJ, Madsen R. Effect of desflurane anesthesia on transcortical motor evoked potentials. J Neurosurg Anesthesiol. 1996;8:47–51.
Kawaguchi M, Inoue S, Kakimoto M, Kitaguchi K, Furuya H, Morimoto T, Sakaki T. The effect of sevoflurane on myogenic motor-evoked potentials induced by single and paired transcranial electrical stimulation of the motor cortex during nitrous xide/ketamine/fentanyl anesthesia. J Neurosurg Anesthesiol. 1998;10:131–6.
Nathan N, Tabaraud F, Lacroix F, Mouliès D, Viviand X, Lansade A, Terrier G, Feiss P. Influence of propofol concentrations on multipulse transcranial motor evoked potentials. Br J Anaesth. 2003;91:493–7.
Furutani K, Deguchi H, Matsuhashi M, Mitsuma Y, Kamiya Y, Baba H. A bolus dose of ketamine reduces the amplitude of the transcranial electrical motor-evoked potential: a randomized, double-blinded, placebo-controlled study. J Neurosurg Anesthesiol. 2019. https://doi.org/10.1097/ana.0000000000000653 (in press, Epub ahead of print).
Deguchi H, Furutani K, Mitsuma Y, Kamiya Y, Baba H. Low-dose droperidol suppresses transcranial electrical motor-evoked potential amplitude: a retrospective study. J Clin Monit Comput. 2021;35:175–81.
Legatt AD, Emerson RG, Epstein CM, MacDonald DB, Deletis V, Bravo RJ, López JR. ACNS guideline: transcranial electrical stimulation motor evoked potential monitoring. J Clin Neurophysiol. 2016;33:42–50.
Concas A, Santoro G, Mascia MP, Serra M, Sanna E, Biggio G. The general anesthetic propofol enhances the function of gamma-aminobutyric acid-coupled chloride channel in the rat cerebral cortex. J Neurochem. 1990;55:2135–8.
Hara M, Kai Y, Ikemoto Y. Propofol activates GABAA receptor-chloride ionophore complex in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology. 1993;79:781–8.
Grasshoff C, Antkowiak B. Propofol and sevoflurane depress spinal neurons in vitro via different molecular targets. Anesthesiology. 2004;101:1167–76.
Jin YH, Zhang Z, Mendelowitz D, Andresen MC. Presynaptic actions of propofol enhance inhibitory synaptic transmission in isolated solitary tract nucleus neurons. Brain Res. 2009;1286:75–83.
Baars J, Dangel C, Herold K, Hadzidiakos D, Rehberg B. Suppression of the human spinal H-reflex by propofol: a quantitative analysis. Acta Anaesthesiol Scand. 2006;50:193–200.
Kammer T, Rehberg B, Menne D, Wartenberg H-C, Wenningmann I, Urban BW. Propofol and sevoflurane in subanesthetic concentrations act preferentially on the spinal cord: evidence from multimodal electrophysiological assessment. Anesthesiology. 2002;97:1416–25.
Kakinohana M, Fuchigami T, Nakamura S, Kawabata T, Sugahara K. Propofol reduces spinal motor neuron excitability in humans. Anesth Analg. 2002;94:1586–8.
Baars JH, von Dincklage F, Reiche J, Rehberg B. Propofol increases presynaptic inhibition of Ia afferents in the intact human spinal cord. Anesthesiology. 2006;104:798–804.
Kerz T, Hennes H-J, Fève A, Decq P, Filipetti P, Duvaldestin P. Effects of propofol on H-reflex in humans. Anesthesiology. 2001;94:32–7.
Kungys G, Kim J, Jinks SL, Atherley RJ, Antognini JF. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg. 2009;108:1531–7.
von Dincklage F, Reiche J, Rehberg B, Baars JH. H-reflex depression by propofol and sevoflurane is dependent on stimulus intensity. Clin Neurophysiol. 2006;117:2653–60.
Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14.
Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968;91:15–36.
Lemon RN, Landau W, Tutssel D, Lawrence DG. Lawrence and Kuypers (1968a, b) revisited: copies of the original filmed material from their classic papers in brain. Brain. 2012;135:2290–5.
Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA. 2009;106:918–23.
Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218.
Ueno M, Nakamura Y, Li J, Gu Z, Niehaus J, Maezawa M, Crone SA, Goulding M, Baccei ML, Yoshida Y. Corticospinal circuits from the sensory and motor cortices differentially regulate skilled movements through distinct spinal interneurons. Cell Rep. 2018;23:1286–300.
Deletis V, Rodi Z, Amassian VE. Neurophysiological mechanisms underlying motor evoked potentials in anesthetized humans. Part 2. Relationship between epidurally and muscle recorded MEPs in man. Clin Neurophysiol. 2001;112:445–52.
Leslie K, Clavisi O, Hargrove J. Target-controlled infusion versus manually-controlled infusion of propofol for general anaesthesia or sedation in adults. Cochrane Database Syst Rev. 2008;3:CD006059.
Ohashi M, Watanabe K, Furutani K, Hirano T, Katsumi K, Shoji H, Mizouchi T, Endo N. False-negative transcranial motor evoked potentials (TcMEPs) during surgery for congenital lumbar kyphoscoliosis: a case report. Spinal Cord Ser Cases. 2017;3:17053.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This work was supported by a Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (Grant Number 18K16441).
Author information
Authors and Affiliations
Contributions
All authors contributed to study conception and design. Material preparation, data collection, and analysis were performed by HD, KF, and YM. Project administration was performed by YK, and HB supervised the project. The first draft of the manuscript was written by HD, and all authors commented on versions of the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Deguchi, H., Furutani, K., Mitsuma, Y. et al. Propofol reduces the amplitude of transcranial electrical motor-evoked potential without affecting spinal motor neurons: a prospective, single-arm, interventional study. J Anesth 35, 434–441 (2021). https://doi.org/10.1007/s00540-021-02927-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-021-02927-7