Abstract
Purpose
This clinical trial reports the use of hydroxyethyl starch (HES70/0.55/4) at very high dosages during surgery. HES70/0.55/4 has the lowest molecular weight among all HES products, and thus may have the least side effects. This observational retrospective study clarified the effects of high-dose HES70/0.55/4 on coagulation and renal function up to 1 month after massive bleeding during surgery.
Methods
Of 20875 patients on our surgical database, 31 patients were identified who had lost more than 5000 ml of blood during surgery and had survived for more than 1 month. The fluid balance, and pre- and postoperative laboratory data were analyzed. Patients were assessed using acute kidney injury (AKI) criteria. AKI and non-AKI groups were compared regarding volume of HES70/0.55/4 infused and serum creatinine (Cr) levels before surgery and until 1 month after surgery.
Results
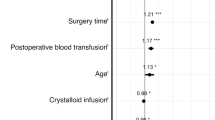
The mean volumes of blood loss, total transfusions, HES70/0.55/4, and urine output during surgery were 8051 ml; 5765 ml; 3085 ml (54 ml/kg); and 1338 ml (2.7 ml/kg/h), respectively. Cr increased, and activated partial thromboplastin time, prothrombin time and international normalized ratio were prolonged postoperatively (0.77–0.9 mg/dl, 34–52 s, and 1.1–1.7, respectively). Of the 31 patients, 13 developed AKI, and 10 of the 13 had recovered at 1 month. Renal impairment due to HES70/0.55/4 was not evident, as shown by the finding that the HES70/0.55/4 amount infused in the AKI patients (53 ml/kg) did not differ from that in the nonAKI patients (55 ml/kg), and there was no relationship between the amount of HES infused and Cr changes.
Conclusion
High-dose HES70/0.55/4 could be safely used in massive bleeding during surgery. HES70/0.55/4 may affect coagulation, but renal impairment was not evident 1 month after surgery.


Similar content being viewed by others
References
Adcock WL, MacGregor A, Davies JR, Hattarki M, Anderson DA, Goss NH. Chromatographic removal and heat inactivation of hepatitis A virus during manufacture of human albumin. Biotechnol Appl Biochem. 1998;28:85–94.
Blümel J, Schmidt I, Willkommen H, Löwer J. Inactivation of parvovirus B19 during pasteurization of human serum albumin. Transfusion. 2002;42:1011–8.
Thyer J, Unal A, Thomas P, Eaton B, Bhashyam R, Ortenburg J, Uren E, Middleton D, Selleck P, Maher D. Prion-removal capacity of chromatographic and ethanol precipitation steps used in the production of albumin and immunoglobulins. Vox Sang. 2006;91:292–300.
Roberts I. (Cochrane Injuries Group Albumin Reviewers) Human albumin administration in critically ill patients: systematic review of randomized controlled trials. Br Med J. 1998;317:235–9.
The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–56.
Johnson ML, Gordon HS, Petersen NJ, Wray NP, Shroyer AL, Grover FL, Geraci JM. Effect of definition of mortality on hospital profiles. Med Care. 2002;40:7–16.
Molitoris BA, Levin A, Warnock DG, Joannidis M, Mehta RL, Kellum JA, Ronco C, Shah SV. Improving outcomes of acute kidney injury: report of an Initiative. Nat Clin Pract Nephrol. 2007;3:439–42.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:31.
Gan T, Bennett GE, Phillips BB, Wakeling H, Moskowitz DM, Olufolabi Y, Konstadt SN, Bradford C, Glass PS, Machin SJ, Mythen MG. Hextend®, a physiologically balanced plasma expander for large volume use in major surgery: a randomized Phase III clinical trial. Anesth Analg. 1999;88:992–8.
Gallandat HRCG, Siemons AW, Bauss D, Rooyen-Butijn WT, Haagenaars JAM, Oeveren W, Bepperling F. A novel hydroxyethyl starch (Voluven®) for effective perioperative plasma volume substitution in cardiac surgery. Can J Anaesth. 2000;47:1207–15.
Westphal M, Jame MFM, Kozek-Langenecker S, Stocker R, Guidet B, Aken HV. Hydoroxyethyl starches. Anesthesiology. 2009;111:187–202.
Yamazaki H. Effects of low-molecular weight HES on kidney—comparison with low-molecular weight dextran (in Japanese with English abstract). Jpn J Anesthesiol. 1975;24:26–43.
Mishler JM. Synthetic plasma volume expanders—their pharmacology, safety and clinical efficacy. Clin Haematol. 1984;13:75–92.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39.
Schortgen F, Lacherade JC, Bruneel F, Cattaneo I, Hemery F, Lemaire F, Brochard L. Effects of hydroxyethyl starch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357:911–6.
Boldt J, Brosch C, Rohm K, Lehmann A, Mengistu A, Suttner S. Is albumin administration in hypoalbuminemic elderly cardiac surgery patients of benefit with regard to inflammation, endothelial activation, and long-term kidney function? Anesth Analg. 2008;107:1496–503.
Treib J, Haass A, Pindur G. Coagulation disorder caused by hydroxyethyl starch. Thromb Haemost. 1997;78:974–83.
Yamakage M, Sato J, Namiki A. Further potential of hydroxyethyl starch-based plasma substitute (in Japanese with English abstract). J Clin Anesth (Japan). 2005;29:61–73.
Yuasa H, Koga Y. Effects of hydroxyethyl starch on hemostasis system (in Japanese with English abstract). J Clin Anesth (Japan). 1998;22:204–8.
Dalrymple-Hay M, Aitchison R, Collins P, Sekhar M, Colvin B. Hydroxyethylstarch induced acquired von Willebrand’s disease. Cin Lab Haematol. 1992;14:209–11.
Conflict of interest
HES70 is a product of Fresenius Kabi Inc. As we found that the second author, Dr. Miyao, had been the medical consultant of this company, we changed the COI form to a new version.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Suzuki, T., Miyao, H., Terui, K. et al. Fluid therapy with hydroxyethyl starch for massive blood loss during surgery. J Anesth 24, 418–425 (2010). https://doi.org/10.1007/s00540-010-0914-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-010-0914-5