Abstract
Background
Anti-tumor necrosis factor (TNF) therapy induces and maintains clinical remission in patients with Crohn’s disease (CD). However, the effect of anti-TNF therapy on the natural course of CD remains controversial. We aimed to investigate the effect of anti-TNF therapy on the initial intestinal surgery for CD.
Methods
In this single-center retrospective cohort study, clinical course of 199 CD patients of inflammatory type at the initial diagnosis (the period between 1973 and 2014) was precisely reviewed until the end of 2016. Patients were divided into TNF and non-TNF groups based on anti-TNF agent use. After comparisons of clinical characteristics and medical treatments, propensity scores were calculated for covariates. Risk of intestinal surgery was compared by a Cox proportional hazards model using the propensity scores. The effect of immunomodulators on initial intestinal surgery was assessed in the TNF group.
Results
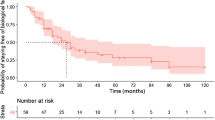
During the study period, 108 patients received anti-TNF therapy. The patients in the TNF group were diagnosed more recently, and more frequently had isolated colonic involvement, and perianal disease. Immunomodulators were more frequently used in the TNF group. Cumulative probability of initial intestinal surgery was significantly lower in the TNF group (P < 0.0001). The hazard ratio in the TNF group was 0.32 (95% CI 0.13–0.74). Immunomodulators did not decrease the risk of initial intestinal surgery.
Conclusions
Anti-TNF therapy can decrease the risk of intestinal surgery among patients with inflammatory-type CD at the initial diagnosis. Further studies should be necessary to determine the additive effect of immunomodulators on the risk of intestinal surgery.



Similar content being viewed by others
Abbreviations
- CD:
-
Crohn’s disease
- TNF:
-
Tumor necrosis factor
- IFX:
-
Infliximab
- ADA:
-
Adalimumab
- IBD:
-
Inflammatory bowel disease
- AZA:
-
Azathioprine
- 6MP:
-
6-Mercaptopurine
- ED:
-
Elemental diet
References
Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–22.
Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg. 2000;231:38–45.
Oriuchi T, Hiwatashi N, Kinouchi Y, et al. Clinical course and longterm prognosis of Japanese patients with Crohn’s disease: predictive factors, rates of operation, and mortality. J Gastroenterol. 2003;38:942–53.
Ramadas AV, Gunesh S, Thomas GAO, et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. 2010;59:1200–6.
Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2014;63:1607–16.
Shi H, Ng S. The state of the art on treatment of Crohn’s disease. J Gastroenterol. 2018;53:989–98.
Targan S, Hanauer S, van Deventer S, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35.
Colombel J, Sandborn W, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Peyrin-Biroulet L, Oussalah A, Williet N, et al. Impact of azathioprine and tumour necrosis factor antagonists on the need for surgery in newly diagnosed Crohn’s disease. Gut. 2011;60:930–6.
Mao E, Hazlewood G, Kaplan G, et al. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalization and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13.
Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106–13.
Jeuring S, van den Heuvel T, Liu L, et al. Improvements in the long-term outcome of Crohn’s disease over the past two decades and the relation to changes in medical management: results from the population-based IBDSL cohort. Am J Gastroenterol. 2017;112:325–36.
Satsangi J. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53.
Peyrin-Biroulet L, Loftus E Jr, Colombel J, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut. 2010;59:141–7.
Rosenbaum P, Rubin DA. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55.
Rosenbaum P, Rubin D. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 2012;79:516–24.
Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–50.
Olaison G, Smedh K, Sjödahl R. Natural course of Crohn’s disease after ileocolic resection: endoscopically visualised ileal ulcers preceding symptoms. Gut. 1992;33:331–5.
De Cruz P, Kamm MA, Prideaux L, et al. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2012;18:758–77.
Renna S, Cammà C, Modesto I, et al. Meta-analysis of the placebo rates of clinical relapse and severe endoscopic recurrence in postoperative Crohn’s disease. Gastroenterology. 2008;135:1500–9.
Frolkis A, Lipton D, Fiest K, et al. Cumulative incidence of second intestinal resection in Crohn’s disease: a systematic review and meta-analysis of population-based studies. Am J Gastroenterol. 2014;109:1739–48.
Rutgeerts P, Diamond R, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63:433–42.
Rutgeerts P, Van Assche G, Sandborn W, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–11.
Walters T, Kim M, Denson L, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn’s disease. Gastroenterology. 2014;146:383–91.
D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomized trial. Lancet. 2008;371:660–7.
Ferrante M, Colombel J, Sandborn W, et al. Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145:978–86.
Shah S, Colombel J, Sands B, et al. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment Pharmacol Ther. 2016;43:317–33.
Burisch J, Pedersen N, Cukovic-Cavka S, et al. Initial disease course and treatment in an inflammatory bowel disease inception cohort in europe. Inflamm Bowel Dis. 2014;20:36–46.
Ng S, Zeng Z, Niewiadomski O, et al. Early Course of Inflammatory Bowel Disease in a Population-based Inception Cohort Study from 8 Countries in Asia and Australia. Gastroenterology. 2016;150:86–95.
Colombel J, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152:351–61.
Lichtenstein G, Feagan B, Cohen R, et al. Drug therapies and the risk of malignancy in Crohn’s disease: results From the TREAT registry. Am J Gastroenterol. 2014;109:212–23.
Beaugerie L, Carrat F, Colombel J, et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut. 2014;63:1416–23.
Ma C, Beilman C, Huang V, et al. Anti-TNF therapy within 2 Years of Crohn’s disease diagnosis improves patient outcomes: a retrospective cohort study. Inflamm Bowel Dis. 2016;22:870–9.
Peyrin-Biroulet L, Harmsen W, Tremaine W, et al. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970–2004). Am J Gastroenterol. 2012;107:1693–701.
Colombel J, Sandborn W, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95.
Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis. 2016;10:1259–66.
Nakase H, Motoya S, Matsumoto T, et al. Significance of measurement of serum trough level and anti-drug antibody of adalimumab as personalized pharmacokinetics in patients with Crohn’s disease: a subanalysis of the DIAMOND trial. Aliment Pharmacol Ther. 2017;46:873–82.
Allez M, Lemann M, Bonnet J, et al. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy1. Am J Gastroenterol. 2002;97:947–53.
Acknowledgements
The authors received generous statistical support from J. Kishimoto, a statistician at Kyushu University, Fukuoka, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Motohiro Esaki received research grants from, Mitsubishi Tanabe Pharma (MTP), EA Pharma (EAP), AbbVie GK (AGK), ZERIA Pharma (ZP), and Asahi Kasei Medical (AKM), and lecture fee from MTP and AGK. Takayuki Matsumoto received research grants from MTP, EAP, and Nippon Kayaku, and lecture fee from MTP, EAP, KYORIN Pharma, AGK, Janssen Pharma, Mochida Pharma, Pfizer, ZP, and AstraZeneca, and advisory fee from JIMRO, Kissei Pharma, and Takeda Pharma (TP). Takanari Kitazono received research grants from Daiichi Sankyo (DS), TP, MTP, Astellas Pharma, Chugai Pharma, MSD, Boehringer Ingelheim, Bristol-Myers, Kyowa Hakko Kirin, AKM, Otsuka Pharma, and Torii Pharma, and lecture fee from DS. The remaining authors disclose no conflicts of interest. This study did not receive any financial support.
Rights and permissions
About this article
Cite this article
Nagata, Y., Esaki, M., Moriyama, T. et al. Anti-tumor necrosis factor therapy decreases the risk of initial intestinal surgery after diagnosis of Crohn’s disease of inflammatory type. J Gastroenterol 54, 330–338 (2019). https://doi.org/10.1007/s00535-018-1511-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1511-x