Abstract
Several new developments have occurred in the field of pancreatic neuroendocrine neoplasm (PNEN) recently in Japan. First, the utility of chromogranin A (CgA), useful for the diagnosis and monitoring of the treatment response of neuroendocrine neoplasm (NEN), has been demonstrated in Japan. For PNEN diagnosis and treatment, grading and correct histological diagnosis according to the WHO 2010 classification is important. Regarding the histological diagnosis, the advent of endoscopic ultrasonography-guided fine-needle aspiration (EUS-FNA) has enabled correct pathological diagnosis and suitable treatment for the affected tissue. Furthermore, EUS-FNA has also facilitates the assessment of the presence or absence of gene mutations. In addition, patients who have a well-differentiated neuroendocrine tumor (NET) showing a Ki-67 index of higher than 20 % according to the WHO 2010 classification, have also been identified, and their responses to treatment were found to be different from those of patients with poorly differentiated neuroendocrine carcinoma (NEC). Therefore, the concept of NET G3 was proposed. Additionally, somatostatin receptor type 2 is expressed in several cases of NET, and somatostatin receptor scintigraphy (111In-octreoscan) has also been approved in Japan. This advancement will undoubtedly contribute to the localization diagnosis, the identification of remote metastasis, and assessments of the treatment responses of PNEN. Finally, regarding the treatment strategy for PNEN, the management of liver metastasis is important. The advent of novel molecular-targeted agents has dramatically improved the prognosis of advanced PNEN. Multimodality therapy that accounts for the tumor stage, degree of tumor differentiation, tumor volume, and speed of tumor growth is required.
Similar content being viewed by others
Introduction
There have been numerous developments with respect to pancreatic neuroendocrine neoplasms (PNENs) during the previous few years in Japan. In this article, we will review advances in the diagnosis and treatment of PNENs in Japan. Firstly, while the benefit of chromogranin A (CgA) in the diagnosis and monitoring of treatment response has been demonstrated in Europe and the U.S., CgA is not yet covered under insurance in Japan. However, its benefit has been recently reported in Japan [1]. Therefore, we will discuss the benefits and limitations of CgA. Next, the grading and correct histological diagnosis according to the WHO 2010 classification is important in the diagnosis and treatment of PNEN [2]. In addition, correct assessment of the presence or absence of functionality, grading, the identification of any metastasis, and the establishment of a treatment suitable for the degree of differentiation and malignancy of the tumor, are necessary [3]. Owing to the ready availability of endoscopic ultrasonography (EUS), EUS-guided fine-needle aspiration (EUS-FNA) can now be performed on pancreatic tumors, which were managed using observation and followed-up previously. Thus, a correct pathological diagnosis and the delivery of a treatment suitable for the tissue have become possible [4, 5]. Meanwhile, although NEN with a Ki-67 index >20 % is classified as neuroendocrine carcinoma (NEC) according to the WHO 2010 classification, cases of well-differentiated neuroendocrine tumor (NET) with a Ki-67 index >20 %, exhibiting different treatment response, have also been identified recently [6]. Therefore, the concept of NET G3 was proposed, and has been included in the draft revision of the WHO classification for 2016. Furthermore, somatostatin receptor (SSTR) type 2 is expressed in numerous cases of NENs, and somatostatin receptor scintigraphy (SRS) [7] that utilizes the expression of SSTR type 2 has also been approved in Japan. The method involves the use of indium pentetreotide (111In), and will undoubtedly contribute to localization diagnosis, the identification of remote metastasis, and the assessment of treatment response in patients with PNEN. Meanwhile, the therapeutic approach to PNEN associated with liver metastasis is changing [8]. Even in cases with liver metastases, surgical treatment is the standard therapy if curative resection is possible. However, because recently developed molecular-targeted agents, such as everolimus and sunitinib, and streptozotocin (STZ) have now become covered under insurance in Japan, multimodality therapy that takes into consideration the condition of the patient, degree of tumor differentiation, tumor volume, and speed of tumor growth, is required [9]. Although prior to the advent of molecularly target therapy, liver metastasis was considered as a main prognosis factor of PNEN, currently the identification of a method by which to control liver metastasis is critical in order to improve the prognosis of patients with PNEN [8].
Benefits and limitations of chromogranin A in pancreatic neuroendocrine neoplasm
CgA is an acidic glycoprotein consisting of 439 amino acids [10–12]. CgA is stored in the secretory granules in neuroendocrine cells (together with various hormones) and is released when the granules are stimulated [12–14]. CgA is widely used as an immunohistochemical marker for PNEN. However, currently in Japan, the measurement of serum CgA levels is not covered under insurance. In Europe and the U.S., the usefulness of CgA as a serum marker has been established, and CgA is used clinically [15–17]. Recently, the efficacy of CgA as a serum marker in patients with PNEN was demonstrated in Japan [1] (Fig. 1). Immunohistologically, it is known that the positive rate of CgA is high in NET G1/G2, and low in NEC [2]. With regard to the degree of differentiation, it is reported that serum CgA levels are significantly higher in NET G2 than in NET G1 [18], while further studies have indicated that there is no significant difference between NET G1 and G2 [1, 19]. Similar to the immunohistological tendencies, the serum CgA level is reported to be lower in NEC than in NET G1/G2. In addition, a number of studies have demonstrated that the sensitivity of serum CgA in a functioning tumor is higher than that in a non-functioning tumor [20, 21]. Furthermore, it has been reported that the serum CgA level increases as the tumor volume increases [1, 18, 21]. Thus, serum CgA levels are typically elevated in patients with larger tumors and in those who have remote metastasis [1, 18, 21]. Therefore, multiple studies have shown that the measurement of serum CgA levels is useful for assessing treatment response and for predicting tumor recurrence [1, 18]. Antacids and steroids are drugs that affect the measured levels of CgA, and among them, proton pump inhibitors (PPIs) require the most attention [1, 22, 23]. PPIs increase the serum CgA levels through the proliferation of enterochromaffin cells [1, 23, 24]. The serum CgA levels are elevated by approximately 2.5 times following the consumption of an oral PPI. Therefore, it is desirable to measure the serum CgA levels 2 weeks after interrupting oral PPI administration [24]. However, in cases of gastrinoma exhibiting Zollinger–Ellison syndrome involving multiple ulcers, the patient’s conditions can rapidly be exacerbated by the discontinuation of oral PPI depending on the case; therefore, stopping oral PPI administration is not recommended. Serum CgA levels are elevated in various illnesses; among them, renal impairment warrants particular attention [25]. Serum CgA levels increase with decreasing creatinine clearance [25]. Other neoplastic diseases, such as prostate cancer and pheochromocytoma, also result in the elevation of serum CgA levels [10, 12]. It is important to note the findings of a study reporting that chronic pancreatitis and pancreatic cancer, which are sometimes difficult to diagnose differentially from PNEN, also elevate CgA levels [26]. However, according to our study, the serum CgA levels in chronic pancreatitis and pancreatic cancer without use of PPI were similar to those in healthy patients [1] (Fig. 1).
Distribution of serum chromogranin A (CgA) level in patients with various pancreatic diseases with or without proton pump inhibitor (PPI) use. Modified from reference [1] by Hijioka et al. The mean serum CgA level of patients with PNET was 6.5-fold higher than normal controls (NC) and was significantly higher compared with controls (p < 0.01). It was also 4.5-fold higher than those of other groups and was significantly higher compared with those of pancreatic cancer (PC) and chronic pancreatitis (CP) groups (p < 0.05). In a subgroup analysis based on PPI use, the serum CgA level of patients with PPI use was higher than that of patients without PPI use in patients in the PC, CP, and autoimmune pancreatitis (AIP) groups with significance (p < 0.05, respectively) but not in the PNET group. In patients without PPI use, the mean serum CgA level of patients with PNET was 7.1-fold higher than NC which was significantly different from NC and patients with PC group (p < 0.01, p < 0.05, respectively) but not from patients with CP and AIP group. Furthermore, the serum CgA levels of the patients with PC, CP, and AIP group not taking PPI was not different from controls. Asterisk PPI was not used in normal control group
EUS-FNA in pancreatic neuroendocrine neoplasm
EUS-FNA has become an essential assessment for the qualitative diagnosis of pancreatic neoplasms. The role of EUS-FNA in the diagnosis of PNEN is two-fold. Its first role is to identify pancreatic neoplasms by EUS and to correctly diagnose PNEN. Its second role is to diagnose the degree of malignancy according to the WHO 2010 classification [2] using the Ki-67 index and the mitotic index to aid the development of a treatment strategy once the condition has been diagnosed as PNEN [3, 5].
EUS-FNA has been used widely since it became covered under insurance in Japan in 2010. With regard to histological diagnosis, the final diagnosis includes cytological diagnosis in addition to the immunostaining of CgA and synaptophysin. The sensitivity of this method is reported to be between 82.6 and 100 %, and the diagnostic accuracy is between 83.3 and 93 % [28–32]. With regard to factors affecting diagnostic accuracy, NET that shows >30 % fibrosis in the maximum cut surfaces of the lesion in the head of the pancreas and the resected specimen is reported to constitute a factor that makes diagnosis difficult [28]. Even when a sufficient amount of samples can be collected by EUS-FNA, there is a possibility that diagnosis is made incorrectly. Many of the reported cases are those in which differential diagnosis from solid and pseudopapillary neoplasm (SPN) is difficult [28, 33]. In SPN, synaptophysin, an endocrine marker, often becomes positive, and CgA may also become positive. Particularly, when a pseudopapillary structure, which is characteristic to SPN, has disappeared due to crush, morphologies become similar, and therefore care should be taken. When submitting specimens, the clinical side should actively request differential diagnosis.
Some studies have discussed the malignancy classification according to the WHO classification 2010 and the diagnostic ability of malignancy by EUS-FNA. According to a recent systematic review, the concordance rate is not necessarily high (83 %) [34]. The reasons for this rather low concordance rate are varied. With regard to the method for Ki-67 measurement, there are many factors that affect counting, such as problems in formalin fixation, methods for immunostaining, and methods of cell counting. As a factor on the part of the tumor, intratumor heterogeneity of Ki-67 is also a problem (Fig. 2). That the so-called hot spot is not collected due to the homogeneity in the tumor is considered to be a reason for the low concordance rate. Therefore, to raise the concordance rate as much as possible, it is important to collect not less than 2000 tumor cells from the EUS-FNA sample as recommended by the European Neuroendocrine Tumor Society (ENETS) [4]. In addition, Fujimori et al. [35] reported that while the overall concordance rate of malignancy was 69.2 %, when a cutoff value of 20 mm for tumor diameter was applied, the concordance rate of tumors with a diameter of <20 mm was 87.5 %, and that of tumors with a diameter of ≥20 mm was 57.1 %. Unno et al. [36] also investigated the possible association with tumor diameter, and reported that concordance was obtained in all patients with tumors with a diameter of <18 mm. Hijioka et al. also reported that the diagnostic accuracy for PNEN with a diameter of <10 mm was 100 %, and proposed that the reason for this due to low fibrosis and high cell density [5]. Thus, one of the reasons for this high concordance may be because tumors with a smaller diameter possess less heterogeneity.
Distribution of the Ki-67 index in pancreatic neuroendocrine tumors (PNET). Modified from reference [4] by Hasegawa et al. Immunohistochemical staining for Ki-67 was performed on resected specimen from PNET, and the slides were digitally scanned using Scan Scope XT (Aperio Technologies, Vista, CA, USA). Variation of Ki-67 index was observed in different area. The high spot of Ki-67 in this specimen revealed 8.2 %, therefore the diagnosis of grading from WHO criteria 2010 is NET G2
The concept of well-differentiated NET G3 and future perspectives
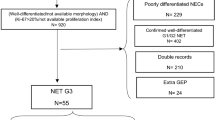
The WHO classification was revised in 2010, and since then, the revised classification has become the standard and has been widely used [2]. However, recently, several issues that the WHO classification does not account for have emerged. These issues are observed in cases of well-differentiated G3 (NET G3) in which the Ki-67 index exceeds 20 % despite its high proliferation rate, and for which a new subclassification is being investigated. According to the WHO 2010 classification, poorly differentiated neuroendocrine carcinoma (PDNEC) is included in NEC, and its prognosis is extremely poor compared to well-differentiated NET G1/G2 [37]. Due to the fact that NEC has a different molecular biology than NET G1/G2 in terms of gene mutations, and resembles small cell lung cancer [5, 38], platinum-based chemotherapy is used as the standard treatment for NEC, as in the case in the treatment of small cell lung cancer [27]. However, it has become clear that there are differences in terms of clinical characteristics between NET G3 and NEC; for example, the proliferative activity of NET G3 is lower than that of NEC, and the prognosis of NET G3 is superior to that of NEC. Furthermore, the response rates observed with the use of platinum agents for the treatment of NET G3 is reported to be lower than that of NEC [39]. Thus, the therapeutic approaches to NET G3 and NEC should be different. Although the morphologies of NET G1/G2 and NEC are distinct, the following points should be taken into consideration when pathologically diagnosing NET G3 and NEC. Firstly, when cell proliferative activity is low despite the histological morphology indicating NEC, the fixation or staining conditions should be reviewed and the credibility of Ki-67 immunostaining should be reconfirmed. Furthermore, there are cases in which NET G3 is erroneously judged to be NEC because of irregular nuclear contours and nuclear concentrations that are conspicuous and because the tissue construction is not clearly observed due to crushes and artefacts at the time of specimen collection. Thus, even in cases in which NEC is suspected based on the examination of small samples such as biopsy specimens or samples with insufficient fixation, cautious judgment should be made in reference to the results of Ki-67 and mitotic indexes. Furthermore, NET G3 may include, albeit rarely, pleomorphic variants with strong cellular pleomorphism and low cell proliferative activity. Therefore, it is important not only to consider the strength of cellular atypia but also to cautiously observe cell density, tissue construction, and the presence or absence of necrosis [40]. In the 2016 ENETS guidelines, PNEN with a Ki-67 index of >20 % is generally referred to as NEN G3 and a treatment algorithm has been prepared by clearly classifying NEN G3 into NET G3 and NEC G3 [41]. A revision of the WHO classification is intended. Regarding PNEN, a draft plan (as shown in Table 1) has been proposed; however, the formal revised edition remains pending.
Somatostatin receptor scintigraphy in pancreatic neuroendocrine neoplasm
SRS was approved for use in January 2016 in Japan, and is beneficial for identifying the distribution of NET throughout the body. This method may be explained as follows: octreotide, a somatostatin analog containing pentetreotide labeled with radioactive indium (111In), is intravenously administered. Subsequently, the labeled compound specifically binds with and accumulates in SSTR to make imaging of NET possible. Concomitant use of single photon emission computed tomography (SPECT) is useful for improving the detection rate of lesions. Recently, 68Ga-DOTATOC and 68Ga-DOTATATE have been used, and are reported to be useful for detecting rate of lesions [7]. One case using 68Ga-DOTATOC-SRS was shown in Fig. 3. Case 1; a female who underwent surgery for duodenal gastrinoma 2 years ago. 68Ga-DOTATOC-PET/CT scan (a–c) was performed for suspected recurrence due to her high gastrin level. Conventional imaging modalities, such as CT and fluorodeoxyglucose positron emission tomography (FDG-PET)/CT, had been negative, but 68Ga-DOTATOC-PET/CT scan demonstrated a focal intense uptake close to the lateral segment of the liver, indicating metastasis (a–c, arrows). Retrospectively, a small lymph node with early enhancement was identified on enhanced CT (d, arrow). A nodal metastasis was confirmed by surgery. The diagnostic ability of SRS is superior to the combined use of computed tomography (CT) and magnetic resonance imaging (MRI) in terms of sensitivity, specificity, and diagnostic accuracy [42]. In addition, a study reported that SRS in combination with CT and MRI enabled the detection of postoperative recurrence 15.5 months earlier than that by the combined use of CT and MRI [42]. For cases of PNEN, SRS is used for the purpose of (1) diagnosis of localization and metastasis [43, 44], (2) confirmation of SSTR2 expression [45, 46], and (3) follow-up [42, 47]. Several cases have been described, including the following: case 2, in which the serum gastrin levels increased after surgery for a gastrinoma, however, recurrence was suspected, and the recurrent lesion was not confirmed by CT (Fig. 4a). 111In-SRS, accumulation was observed in the abdominal lymph node (Fig. 4b), and thus recurrence in the lymph node was diagnosed. In case 3, the patient had a non-functioning PNET with liver metastases, and 111In-SRS was observed to have accumulated in the primary focus in the pancreatic head and liver metastatic lesions (Fig. 5a–d). However, by the use of 111In-SRS, bone metastasis, which was not detected by CT, was found (Fig. 5e). The identification of the primary focus and the metastatic focus of PNEN is not sufficiently performed by CT alone, and FDG-PET is also conducted for the purpose of a whole-body search for NEN. However, because glucose metabolism is not enhanced in a number of NET G1/G2 lesions, its detection is complex. By contrast, with regard to NEC G3, uptake in FDG-PET is observed in numerous cases; therefore, FDG-PET in combination with 111In-SRS could be useful for the differential diagnosis of NEC G3 and NET G3. There is, however a limitation to the detection capability of SRS. In particular, the expression of SSTR2 in insulinoma is low (approximately <50 %), and thus may not be detected by SRS in certain cases. In such cases, the use of glucagon-like peptide-1 analogue (exendin-4) receptor imaging has recently been reported [48, 49]. Its sensitivity is high (approximately 95 %).
Case 1 underwent surgery for duodenal gastrinoma. 68Ga-DOTATOC-SRS scan (a–c) was performed for suspected recurrence due to her high gastrin level. Conventional imaging modalities, such as CT and FDG-PET, had been negative, but 68Ga-DOTATOC-SRS demonstrated a focal intense uptake close to the lateral segment of the liver, indicating metastasis (a–c, arrows). Retrospectively, a small lymph node with early enhancement was identified on enhanced CT (d, arrow). A nodal metastasis was confirmed by surgery
Treatment approach to pancreatic neuroendocrine neoplasm with liver metastasis
There had previously been no effective treatment methods for the treatment of PNEN with liver metastasis until novel molecular target agents became available. Molecular target agents have been reported to be effective in Japanese patients with PNET [50, 51]; however, the long-term prognosis of PNEN in the Japanese population remains unknown. Recently, we conducted a retrospective study to establish the long-term prognosis and prognostic factors in Japanese patients with advanced PNEN [8]. The study enrolled 78 patients with advanced PNEN, of whom 74 % had non-functioning tumors. The cases of 13 patients were complicated by hereditary diseases, of which 11 patients had multiple endocrine neoplasia (MEN) type-1. Liver metastasis was observed in 83 % of the patients, 54 patients of whom had multiple (five or more) lesions. To examine the benefit of various clinical and histological factors as potential prognostic factors in patients with advanced PNEN, a univariate analysis was performed. This analysis showed that overall survival (OS) in patients with bone metastasis was significantly low (HR, 4.38; 95 % CI 1.42–11.29; p = 0.013). Furthermore, when the cases were classified into groups of before and after the introduction of novel molecular target agents, the prognosis in patients treated after the introduction of novel molecularly targeted drugs was significantly improved (HR, 0.07; 95 % CI 0.03-0.19; p < 0.001) (Fig. 6). In multivariate analysis based on factors for which the p values were 0.20 or lower in the univariate analysis, a Ki-67 index cutoff value of 10 % (HR, 38.8; 95 % CI 8.42–226.62; p < 0.001), bone metastasis (HR, 5.66; 95 % CI 1.10–24.00; p = 0.039), and treatment period before and after the introduction of molecular target therapy (HR, 0.02; 95 % CI 0.00–0.11; p < 0.001) were the independent factors, as in the univariate analysis. Incidentally, the OS in patients with functioning tumors was generally lower than in those with non-functioning tumors (HR, 2.68; 95 % CI 0.98–7.62; p = 0.054), although the difference was not significant [8]. As such, it was shown for the first time that the prognosis of advanced PNET was dramatically improved by the introduction of novel molecular targeted agents. Specifically, the use of everolimus and sunitinib is thought to have contributed greatly to the dramatic improvement in the prognosis for advanced PNEN in Japanese patients. In addition, because of that liver metastasis, which has been considered to be a prognostic factor, has been rendered controllable by molecular targeted agents, the prognostic factors of advanced PNET have altered greatly. The most important feature may be whether the liver metastasis is controllable. Furthermore, with regard to STZ, the analysis of a greater number of cases is necessary. Future studies are warranted in order to examine the type of cases in which STZ will be beneficial, and also the circumstances in which it should be used as a first-line therapy.
Kaplan–Meier analyses of different prognostic factors. Overall survival (OS) in patients with advanced PNEN according to treatment timing before and after arrival of molecular target therapy. Modified from reference [8] by Lee et al. A univariate analysis performed with several clinicopathological factors revealed that patients treated after the advent of targeted therapies had significantly better prognosis than those who terminated treatment before their implementation (HR: 0.07, 95 % CI 0.03–0.19, p < 0.001)
Discussion
An epidemiological survey of PNEN was conducted in Japan, and the tangible condition with regard to the disease was clarified [52–55]. Recently, the result of the second national epidemiological survey was reported [54]. In this survey, the number of treated patients for PNEN in 2010 was estimated to be 3379, which showed an increase of approximately 1.2 times of the number in 2005 [53]. Interestingly, non-functioning PNEN accounted for 65.5 % of all PNEN cases in 2010. As expected, the ratio of non-functioning PNEN increased compared to that in 2005. The reason for this change may be attributed to the improvement in histological diagnosis as a result of the recent widespread availability of EUS-FNA. As previously mentioned, the WHO classification was revised in 2010 [2]. In the second survey, the ratio of NEC according to the WHO 2010 classification was reported for the first time in Japan. In the survey, the NEC ratio was 7.5 % of all the patients with PNEN. Subsequent to a diagnosis of PNEN, remote metastasis was observed in 12.9 % of patients with NET G1/G2; however, it was observed in as many as 46.3 % of patients with NEC. Currently, in cases in which the Ki-67 index exceeds 20 %, yet the neoplasm remains well differentiated, treatment response is different to that of NEC G3 [39]. Thus, an attempt to create a new category within NET G3 is underway, and as a consequence, the classification in future epidemiological surveys will need to be altered.
Recently, Ohki et al. discovered PHLDA3, a new cancer suppressor gene in PNET [56]. PHLDA3 is a target gene of p53, which was discovered when the binding of p53 protein with the genome and modified histone were analyzed by ChIP-chip and CHIP-seq methods [57]. Most PNET is associated with the activation of oncogene Akt. It was found that PHLDA3 suppresses the Akt activity by inhibiting the cell membrane transport of Akt in PNEN. In addition, PNET with loss of heterozygosity (LOH) in the PHLDA3 gene showed high malignancy and poor prognosis [56]. As the LOH in the PHLDA3 gene induces the activation of Akt, it is expected that everolimus, an mTOR inhibitor of the Akt pathway, is effective in patients with PNET in whom LOH in the PHLDA3 gene is observed. Furthermore, it may be possible to establish an individualized treatment in such patients [56]. Recently, a randomized study (CLARINET study) of lanreotide, a somatostatin analog, was conducted in patients with SRS-positive, non-functioning PNET and gastrointestinal NET (including those with unknown primary foci) in which the Ki-67 index was <10 % and, which were diagnosed as moderately to well-differentiated neoplasms. A 96-week administration period of lanreotide significantly prolonged the progression-free survival, and thus, the inhibitory effect of lanreotide on tumor growth was demonstrated [58]. A phase II study of lanreotide is currently being conducted in Japan. Lanreotide will be expected to suffice as a further treatment option for unresectable well-differentiated PNET [59].
PNEN are becoming an increasing clinical problem because the number of patients has been increasing and PNETs can frequently present with advanced disease that requires distinct diagnostic and treatment approaches in Japan [60]. Recently, the clinical guidelines for gastroenteropancreatic neuroendocrine tumors in Japan have been published by Japan Neuroendocrine Tumor Society (JNETS) in 2015 [61]. JNETS guideline describes in detail the strategies for diagnosis for PNEN, determinants of resection for PNEN and the management and treatment of patients with advanced metastatic PNEN. We hope this review of will contribute on the diagnostic and treatment approaches for PNEN.
References
Hijioka M, Ito T, Igarashi H, et al. Serum chromogranin A is a useful marker for Japanese patients with pancreatic neuroendocrine tumors. Cancer Sci. 2014;105:1464–71.
Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system, vol. 3. 4th ed. Lyon: IARC; 2010.
Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol. 2012;47:941–60.
Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32–8.
Hijioka S, Hosoda W, Mizuno N, et al. Does the WHO 2010 classification of pancreatic neuroendocrine neoplasms accurately characterize pancreatic neuroendocrine carcinomas? J Gastroenterol. 2015;50:564–72.
Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60.
Ito T, Jensen RT. Imaging in multiple endocrine neoplasia type 1: recent studies show enhanced sensitivities but increased controversies. Int J Endocr Oncol. 2016;3:53–66.
Lee L, Igarashi H, Fujimori N, et al. Long-term outcomes and prognostic factors in 78 Japanese patients with advanced pancreatic neuroendocrine neoplasms: a single-center retrospective study. Jpn J Clin Oncol. 2015;45:1131–8.
Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol. 2012;26:737–53.
Singh S, Law C. Chromogranin A: a sensitive biomarker for the detection and post-treatment monitoring of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2012;6:313–34.
Ito T, Igarashi H, Jensen RT. Serum pancreastatin: the long sought universal, sensitive, specific tumor marker for neuroendocrine tumors? Pancreas. 2012;41:505–7.
Modlin IM, Gustafsson BI, Moss SF, Pavel M, Tsolakis AV, Kidd M. Chromogranin A-biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–43.
Konecki DS, Benedum UM, Gerdes H-H, Huttner WB. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987;262:17026–30.
Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N. Engl J Med. 2003;348:1134–49.
Öberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–81.
Campana D, Nori F, Piscitelli L, et al. Chromogranin A: is it a useful marker of neuroendocrine tumors? J Clin Oncol. 2007;25:1967–73.
O’Toole D, Grossman A, Gross D, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: biochemical markers. Neuroendocrinology. 2009;90:194–202.
Paik WH, Ryu JK, Song BJ, et al. Clinical usefulness of plasma chromogranin a in pancreatic neuroendocrine neoplasm. J Korean Med Sci. 2013;28:750–4.
Korse CM, Taal BG, Vincent A, et al. Choice of tumour markers in patients with neuroendocrine tumours is dependent on the histological grade. A marker study of chromogranin A, neuron specific enolase, progastrin-releasing peptide and cytokeratin fragments. Eur J Cancer. 2012;48:662–71.
Baudin E, Gigliotti A, Ducreux M, et al. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br J Cancer. 1998;78:1102–7.
Nehar D, Lombard-Bohas C, Olivieri S, et al. Interest of chromogranin A for diagnosis and follow-up of endocrine tumours. Clin. Endocrinol. (Oxf). 2004;60:644–52.
Sanduleanu S, De Bruïne A, Stridsberg M, et al. Serum chromogranin A as a screening test for gastric enterochromaffin-like cell hyperplasia during acid-suppressive therapy. Eur J Clin Invest. 2001;31:802–11.
Mosli HH, Dennis A, Kocha W, et al. Effect of short-term proton pump inhibitor treatment and its discontinuation on chromogranin a in healthy subjects. J Clin Endocrinol Metab. 2012;97:E1731–5.
Korse CM, Muller M, Taal BG. Discontinuation of proton pump inhibitors during assessment of chromogranin A levels in patients with neuroendocrine tumours. Br J Cancer. 2011;105:1173–5.
Bech PR, Ramachandran R, Dhillo WS, et al. Quantifying the effects of renal impairment on plasma concentrations of the neuroendocrine neoplasia biomarkers chromogranin A, chromogranin B, and cocaine- and amphetamine-regulated transcript. Clin Chem. 2012;58:941–3.
Malaguarnera M, Cristaldi E, Cammalleri L, et al. Elevated chromogranin A (CgA) serum levels in the patients with advanced pancreatic cancer. Arch Gerontol Geriatr. 2009;48:213–7.
Ikeda M, Okuyama H, Takahashi H, et al. Chemotherapy for advanced poorly differentiated pancreatic neuroendocrine carcinoma. J Hepatobiliary Pancreat Sci. 2015;22:623–7.
Hijioka S, Hara K, Mizuno N, et al. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol. 2016. doi:10.1007/s00535-016-1164-6
Ginès A, Vazquez-Sequeiros E, Soria MT, et al. Usefulness of EUS-guided fine needle aspiration (EUS-FNA) in the diagnosis of functioning neuroendocrine tumors. Gastrointest Endosc. 2002;56:291–6.
Ardengh JC, de Paulo GA, Ferrari AP. EUS-guided FNA in the diagnosis of pancreatic neuroendocrine tumors before surgery. Gastrointest Endosc. 2004;60:378–84.
Figueiredo FAF, Giovannini M, Monges G, et al. EUS-FNA predicts 5-year survival in pancreatic endocrine tumors. Gastrointest Endosc. 2009;70:907–14.
Pais SA, Al-Haddad M, Mohamadnejad M, et al. EUS for pancreatic neuroendocrine tumors: a single-center, 11-year experience. Gastrointest Endosc. 2010;71:1185–93.
Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–72.
Pezzilli R, Partelli S, Cannizzaro R, et al. Ki-67 prognostic and therapeutic decision driven marker for pancreatic neuroendocrine neoplasms (PNENs): a systematic review. Adv Med Sci. 2016;61:147–53.
Fujimori N, Osoegawa T, Lee L, et al. Efficacy of endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors. Scand J Gastroenterol. 2016;51:245–52.
Unno J, Kanno A, Masamune A, et al. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand J Gastroenterol. 2014;49:1367–74.
Velayoudom-Cephise FL, Duvillard P, Foucan L, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20:649–57.
Yachida S, Vakiani E, White CM, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173–84.
Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–60.
Kasajima A, Yazdani S, Sasano H. Pathology diagnosis of pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci. 2015;22:586–93.
Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–71.
Scigliano S, Lebtahi R, Maire F, et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer. 2009;16:977–90.
Bodei L, Sundin A, Kidd M, et al. The status of neuroendocrine tumor imaging: from darkness to light? Neuroendocrinology. 2015;101:1–17.
Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–77.
Eriksson B, Klöppel G, Krenning E, et al. Consensus guidelines for the management of patients with digestive neuroendocrine tumors–well-differentiated jejunal-ileal tumor/carcinoma. Neuroendocrinology. 2008;87:8–19.
Pavel M, O’Toole D, Costa F, et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–85.
Arnold R, Chen YJ, Costa F, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: follow-up and documentation. Neuroendocrinology. 2009;90:227–33.
Christ E, Wild D, Ederer S, et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: a prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013;1(2):115–22.
Xu Y, Pan D, Xu Q, et al. Insulinoma imaging with glucagon-like peptide-1 receptor targeting probe 18F-FBEM–Cys39-exendin-4. J Cancer Res Clin Oncol. 2014;140:1479–88.
Ito T, Okusaka T, Ikeda M, et al. Everolimus for advanced pancreatic neuroendocrine tumours: a subgroup analysis evaluating Japanese patients in the RADIANT-3 trial. Jpn J Clin Oncol. 2012;42:903–11.
Ito T, Okusaka T, Nishida T, et al. Phase II study of sunitinib in Japanese patients with unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumor. Invest New Drugs. 2013;31:1265–74.
Ito T, Tanaka M, Sasano H, et al. Preliminary results of a Japanese nationwide survey of neuroendocrine gastrointestinal tumors. J Gastroenterol. 2007;42:497–500.
Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–43.
Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64.
Ito T, Lee L, Hijioka M, et al. The up-to-date review of epidemiological pancreatic neuroendocrine tumors in Japan. J Hepatobiliary Pancreat Sci. 2015;22:574–7.
Ohki R, Saito K, Chen Y, et al. PHLDA3 is a novel tumor suppressor of pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A. 2014;111:E2404–13.
Kawase T, Ohki R, Shibata T. PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt. Cell. 2009;136:535–50.
Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33.
Igarashi H, Hijioka M, Lee L, et al. Biotherapy of pancreatic neuroendocrine tumors using somatostatin analogs. J Hepatobiliary Pancreat Sci. 2015;22:618–22.
Ito T. Highlights of topic “Pancreatic neuroendocrine tumors update”. J Hepatobiliary Pancreat Sci. 2015;22:573.
Guideline for pancreatic and gastroenteric neuroendocrine tumor. Edited by Japan Neuroendocrine Tumor Society. ISBN: 978-4-307-20339-5, 2015 (in Japanese).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ito, T., Hijioka, S., Masui, T. et al. Advances in the diagnosis and treatment of pancreatic neuroendocrine neoplasms in Japan. J Gastroenterol 52, 9–18 (2017). https://doi.org/10.1007/s00535-016-1250-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1250-9