Abstract
Background
The significance of HBV reactivation during immunosuppressive therapy was evaluated in three nationwide cohorts including patients with previously resolved HBV (prHBV) infection.
Methods
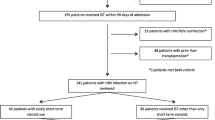
The clinical features of 1061 patients with acute liver failure (ALF) or late-onset hepatic failure (LOHF) were retrospectively examined, focusing on those who experienced HBV reactivation. Additionally, 420 patients with prHBV infection were prospectively enrolled: 203 received immunosuppressive therapies immediately after enrollment, while the remaining 217 were enrolled after having received immunosuppressive therapies without the occurrence of HBV reactivation. The serum HBV-DNA levels were prospectively monitored every month, and the incidences of HBV reactivation, defined as a serum HBV-DNA level of 1.3 log IU/ml or more, were evaluated.
Results
In the retrospective study, persistent HBV infection was found in 90 patients, and HBV reactivation was responsible for liver injuries in 50 patients including 23 receiving immunosuppressive therapies (26 with HBs-antigen positivity, 7 with prHBV infection). None of seven patients with prHBV infection were rescued. In the prospective studies, HBV reactivation occurred in ten patients, but preemptive entecavir administration prevented liver injury. The cumulative reactivation rate was 3.2 % at 6 months, and the increase of the rate compared to that at 6 months was +1.5 % at 48 months.
Conclusions
HBV reactivation during immunosuppression was responsible for liver injuries in a quarter of the ALF/LOHF patients with persistent HBV infection. Early serum HBV-DNA monitoring may improve patient prognosis, since HBV reactivation typically occurs within 6 months of the start of immunosuppressive therapies in patients with prHBV infection.



Similar content being viewed by others
References
World Health Organization. Hepatitis: fact sheet N°204, July 2015. www.who.int/mediacentre/factsheets/fs204/en/.
Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer world wide. J Hepatol. 2006;45:529–38.
Tanaka J, Kumagai J, Katayama K, et al. Sex- and age-specific carriers of hepatitis B and C virus in Japan estimated by the prevalence in the 3,485,648 first-time blood donors during 1995–2000. Intervirol. 2004;47:32–40.
Kusumoto S, Tanaka Y, Mizokami M, et al. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol. 2009;90:13–23.
Wands JR, Chura CM, Roll FJ, et al. Serial studies of hepatitis-associated antigen and antibody in patients receiving antitumor chemotherapy for myeloproliferative and lymphoproliferative disorders. Gastroenterology. 1975;68:105–12.
Lok AS, Liang RH, Chiu EK, et al. Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182–8.
Uemoto S, Sugiyama K, Marusawa H, et al. Transmission of hepatitis B virus from hepatitis B core antibody-positive donors in living related liver transplants. Transplantation. 1998;65:494–9.
Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med. 2001;344:68–9.
Barone M, Notarnicola A, Lopalco G, et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology. 2015;62:40–6.
Urata Y, Uesato R, Tanaka D, et al. Prevalence of reactivation of hepatitis B virus replication in rheumatoid arthritis patients. Mod Rheumatol. 2011;21:16–23.
Mori S. Past hepatitis B infection in rheumatoid arthritis patients receiving biologic and/or nonbiologic disease-modifying antirheumatic drugs. Mod Rheumatol. 2011;21:621–7.
Tamori A, Koike T, Goto H, et al. Prospective study of reactivation of hepatitis B in patients with rheumatoid arthritis who received immunosuppressive therapy: evaluation of both HBsAg-positive and HBsAg-negative cohorts. J Gastroenterol. 2011;46:556–64.
Oketani M, Ido A, Uto H, et al. Prevention of hepatitis B virus reactivation in patients receiving immunosuppressive therapy or chemotherapy. Hepatol Res. 2012;42:627–36.
Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology. JSH guidelines for the management of hepatitis B virus infection. Hepatol Res. 2014;44(Suppl S1):1–58.
Mochida S, Takikawa Y, Nakayama N, et al. Diagnostic criteria of acute liver failure: a report by the intractable hepato-biliary diseases study group of Japan. Hepatol Res. 2011;41:805–12.
Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol. 2012;47:849–61.
Mochida S, Takikawa Y, Nakayama N, et al. Classification of the etiologies of acute liver failure in Japan: a report by the intractable hepato-biliary diseases study group of Japan. Hepatol Res. 2014;44:365–7.
Allice T, Cerutti F, Pittaluga F, et al. COBAS AmpliPrep-COBAS TaqMan hepatitis B virus (HBV) test: a novel automated real-time PCR assay for quantification of HBV DNA in plasma. J Clin Microbiol. 2007;45:828–34.
Fujiwara K, Mochida S, Matsui A, et al. Intractable liver diseases study group of Japan. Fulminant hepatitis and late onset hepatic failure in Japan: summary of 698 patients between 1998 and 2003 analyzed by the annual nationwide survey. Hepatol Res. 2008;38:646–57.
Oketani M, Ide A, Nakayama N, et al. Etiology and prognosis of fulminant hepatitis and late-onset hepatic failure in Japan: summary of the annual nationwide survey between 2004 and 2009. Hepatol Res. 2013;43:97–105.
Reddy KR, Beavers KL, Hammond SP, et al. American gastroenterological association institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–9.
Dickson RC, Terrault NA, Ishitani M, et al. Protective antibody levels and dose requirements for IV 5 % Nabi hepatitis B immune globulin combined with lamivudine in liver transplantation for hepatitis B-induced end stage liver disease. Liver Transpl. 2006;12:124–33.
Knöll A, Boehm S, Hahn J, et al. Long-term surveillance of haematopoietic stem cell recipients with resolved hepatitis B: high risk of viral reactivation even in a recipient with a vaccinated donor. J Viral Hepat. 2007;14:478–83.
Nakamoto S, Kanda T, Nakaseko C, et al. Reactivation of hepatitis B virus in hematopoietic stem cell transplant recipients in Japan: efficacy of nucleos(t)ide analogues for prevention and treatment. Int J Mol Sci. 2014;15:21455–67.
Kusumoto S, Tanaka Y, Suzuki R, et al. Monitoring of hepatitis B virus (HBV) DNA and risk of HBV reactivation in B-Cell lymphoma: a prospective observational study. Clin Infect Dis. 2015;61:719–29.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2.
European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420.
Acknowledgments
We thank the doctors for their cooperation in enrolling patients to Parts 2 and 3 (Supplementary Table). This study was performed with the support of the Ministry of Health, Labour and Welfare as an official project by the Intractable Hepato-biliary Diseases Study Group of Japan and by the Research on Hepatitis and BSE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Satoshi Mochida has received research grants, speaking fees, or honoraria from Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., Dainippon, Mitsubishi Tanabe Pharma Co., MSD K.K., Toray Medical Co. Ltd., Ajinomoto Pharmaceuticals Co. Ltd., and Eisai Co. Ltd. Satoshi Mochida and Yoshihito Uchida have received patent royalties from SRL Inc. Masayoshi Harigai has received research grants from Abbvie Japan Co. Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Co., Ono Pharmaceuticals, Takeda Pharmaceutical Co. Ltd., and UCB Japan. Kenji Ikeda has received speaking fees or honoraria from Sumitomo Dainippon Pharma Co. Ltd. and Eisai Co. Ltd. Masamitsu Nakao, Nobuaki Nakayama, Sumiko Nagoshi, Akio Ido, Toshihide Mimura, Hiroshi Kaneko, Tetsuya Tsuchida, Hiromichi Suzuki, Nobuyuki Ura, Yuichi Nakamura, Masami Bessho, Kazuo Dan, Shigeo Kusumoto, Yasutsuna Sasaki, Hirofumi Fujii, Fumitaka Suzuki, Hiroko Kobayashi, Kazuhiko Yamamoto, Hajime Takikawa, and Hirohito Tsubouchi have no conflict of interest directly relevant to the content of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mochida, S., Nakao, M., Nakayama, N. et al. Nationwide prospective and retrospective surveys for hepatitis B virus reactivation during immunosuppressive therapies. J Gastroenterol 51, 999–1010 (2016). https://doi.org/10.1007/s00535-016-1168-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1168-2