Abstract
Background
The Src homology 2-containing inositol 5-phosphatase 2 (SHIP2) is implicated in diabetes, arthrosclerosis, and cancer. However, the role of SHIP2 in human gastric cancer remains unclear.
Methods
The expression levels of SHIP2 in gastric cancer tissues, a panel of gastric cancer cell lines, and normal gastric epithelial cells were analyzed by immunohistochemistry (IHC), Western blot, and real-time quantitative RT-PCR (qRT-PCR). Gastric cancer cells with either overexpressed SHIP2 or co-overexpressed SHIP2 and Akt were analyzed to determine cell proliferation, colony formation, apoptosis, cell migration, and invasion assays. Normal gastric epithelial cells with knockdown SHIP2 or co-knockdown SHIP2 and Akt were subjected by anchorage-independent growth assays. The effect of SHIP2 on tumor growth in vivo was detected by xenograft tumorigenesis assays.
Results
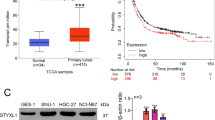
SHIP2 was commonly downregulated in gastric cancer compared with normal gastric mucosa, and overexpression of SHIP2 inhibited cell proliferation, induced apoptosis, suppressed cell motility and invasion in gastric cancer cells in vitro, and retarded the growth of xenograft gastric tumors in vivo, while knockdown of SHIP2 in normal gastric epithelial cells promoted anchorage-independent growth. Moreover, overexpression of SHIP2 inactivated Akt, and upregulated p21, p27, and the pro-apoptotic protein Bim. Restoring Akt activation in gastric cancer cells largely blocked the inhibition of PI3K/Akt signaling by SHIP2 and reversed the inhibitory effect of SHIP2 on tumorigenesis and proliferation.
Conclusions
This study demonstrates, for the first time, that SHIP2 is frequently downregulated in gastric cancer, and reduced SHIP2 expression promotes tumorigenesis and proliferation of gastric cancer via activation of the PI3K/Akt signaling.






Similar content being viewed by others
References
Backers K, Blero D, Paternotte N, et al. The termination of PI3K signalling by SHIP1 and SHIP2 inositol 5-phosphatases. Adv Enzyme Regul. 2003;43:15–28.
Raaijmakers JH, Deneubourg L, Rehmann H, et al. The PI3K effector Arap3 interacts with the PI(3,4,5) P3 phosphatase SHIP2 in a SAM domain-dependent manner. Cell Signal. 2007;19(6):1249–57.
Zhuang G, Hunter S, Hwang Y, et al. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J Biol Chem. 2007;282(4):2683–94.
Huber C, Faqeih EA, Bartholdi D, et al. Exome sequencing identifies INPPL1 mutations as a cause of opsismodysplasia. Am J Hum Genet. 2013;92(1):144–9.
Suwa A, Kurama T, Shimokawa T. SHIP2 and its involvement in various diseases. Expert Opin Ther Targets. 2010;14(7):727–37.
Fu M, Fan W, Pu X, et al. Elevated expression of SHIP2 correlates with poor prognosis in non-small cell lung cancer. Int J Clin Exp Pathol. 2013;6(10):2185–91.
Fu M, Gu X, Ni H, et al. High expression of inositol polyphosphate phosphatase-like 1 associates with unfavorable survival in hepatocellular carcinoma. Int J Clin Exp Pathol. 2013;6(11):2515–22.
Prasad NK, Tandon M, Handa A, et al. High expression of obesity-linked phosphatase SHIP2 in invasive breast cancer correlates with reduced disease-free survival. Tumour Biol. 2008;29(5):330–41.
Yang J, Fu M, Ding Y, et al. High SHIP2 expression indicates poor survival in colorectal cancer. Dis Markers. 2014;2014:218968.
Prasad NK. SHIP2 phosphoinositol phosphatase positively regulates EGFR-Akt pathway, CXCR4 expression, and cell migration in MDA-MB-231 breast cancer cells. Int J Oncol. 2009;34(1):97–105.
Prasad NK, Tandon M, Badve S, et al. Phosphoinositol phosphatase SHIP2 promotes cancer development and metastasis coupled with alterations in EGF receptor turnover. Carcinogenesis. 2008;29(1):25–34.
Fu CH, Lin RJ, Yu J, et al. A novel oncogenic role of inositol phosphatase SHIP2 in ER-negative breast cancer stem cells: involvement of JNK/vimentin activation. Stem Cells. 2014;32(8):2048–60.
Yu J, Ryan DG, Getsios S, et al. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci USA. 2008;105(49):19300–5.
Taylor V, Wong M, Brandts C, et al. 5′ phospholipid phosphatase SHIP-2 causes protein kinase B inactivation and cell cycle arrest in glioblastoma cells. Mol Cell Biol. 2000;20(18):6860–71.
Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Bittoni A, Maccaroni E, Scartozzi M, et al. Chemotherapy for locally advanced and metastatic gastric cancer: state of the art and future perspectives. Eur Rev Med Pharmacol Sci. 2010;14(4):309–14.
Nam SY, Lee HS, Jung GA, et al. Akt/PKB activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS. 2003;111(12):1105–13.
Sasaki T, Kuniyasu H. Significance of AKT in gastric cancer (Review). Int J Oncol. 2014;45(6):2187–92.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19.
Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–44.
Carracedo A, Alimonti A, Pandolfi PP. PTEN level in tumor suppression: how much is too little? Cancer Res. 2011;71(3):629–33.
Ooms LM, Fedele CG, Astle MV, et al. The inositol polyphosphate 5-phosphatase, PIPP, is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol Biol Cell. 2006;17(2):607–22.
Fedele CG, Ooms LM, Ho M, et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci USA. 2010;107(51):22231–6.
Gewinner C, Wang ZC, Richardson A, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16(2):115–25.
Hodgson MC, Shao LJ, Frolov A, et al. Decreased expression and androgen regulation of the tumor suppressor gene INPP4B in prostate cancer. Cancer Res. 2011;71(2):572–82.
Scheid MP, Huber M, Damen JE, et al. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J Biol Chem. 2002;277(11):9027–35.
Ong CJ, Ming-Lum A, Nodwell M, et al. Small-molecule agonists of SHIP1 inhibit the phosphoinositide 3-kinase pathway in hematopoietic cells. Blood. 2007;110(6):1942–9.
Ye Y, Jin L, Wilmott JS, et al. PI(4,5)P2 5-phosphatase a regulates PI3K/Akt signalling and has a tumour suppressive role in human melanoma. Nat Commun. 2013;4:1508.
Colaluca IN, Tosoni D, Nuciforo P, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451(7174):76–80.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501.
Elong Edimo W, Schurmans S, Roger PP, et al. SHIP2 signaling in normal and pathological situations: its impact on cell proliferation. Adv Biol Regul. 2014;54:142–51.
Kisseleva MV, Cao L, Majerus PW. Phosphoinositide-specific inositol polyphosphate 5-phosphatase IV inhibits Akt/protein kinase B phosphorylation and leads to apoptotic cell death. J Biol Chem. 2002;277(8):6266–72.
Vandenbroere I, Paternotte N, Dumont JE, et al. The c-Cbl-associated protein and c-Cbl are two new partners of the SH2-containing inositol polyphosphate 5-phosphatase SHIP2. Biochem Biophys Res Commun. 2003;300(2):494–500.
Prasad N, Topping RS, Decker SJ. SH2-containing inositol 5′-phosphatase SHIP2 associates with the p130 (Cas) adapter protein and regulates cellular adhesion and spreading. Mol Cell Biol. 2001;21(4):1416–28.
Dyson JM, O’Malley CJ, Becanovic J, et al. The SH2-containing inositol polyphosphate 5-phosphatase, SHIP-2, binds filamin and regulates submembraneous actin. J Cell Biol. 2001;155(6):1065–79.
Paternotte N, Zhang J, Vandenbroere I, et al. SHIP2 interaction with the cytoskeletal protein vinexin. FEBS J 2005;272(23):6052–66.
Koch A, Mancini A, El Bounkari O, et al. The SH2-domian-containing inositol 5-phosphatase (SHIP)-2 binds to c-Met directly via tyrosine residue 1356 and involves hepatocyte growth factor (HGF)-induced lamellipodium formation, cell scattering and cell spreading. Oncogene. 2005;24(21):3436–47.
Kato K, Yazawa T, Taki K, et al. The inositol 5-phosphatase SHIP2 is an effector of RhoA and is involved in cell polarity and migration. Mol Biol Cell. 2012;23(13):2593–604.
Xie J, Onnockx S, Vandenbroere I, et al. The docking properties of SHIP2 influence both JIP1 tyrosine phosphorylation and JNK activity. Cell Signal. 2008;20(8):1432–41.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81301738), Anhui Provincial Natural Science Foundation (No. 1408085QH150), Anhui Medical University Training Program of National Outstanding Youth Foundation (No. GJYQ-1402), Research Fund of Anhui Medical University (No. XJ201328), and National Undergraduate Training Programs for Innovation and Entrepreneurship-China (No. 201410366023). We thank David B. Alexander for manuscript review and English checking.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2015_1101_MOESM1_ESM.pdf
This information includes: Table S1 Relationship between SHIP2 expression and clinicopathologic characteristics of GC; Fig. S1 The intracellular localization of SHIP2 in normal gastric mucosa tissues was detected by immunohistochemistry analysis; Fig S2 Expression of SHIP2 in intestinal metaplasia of stomach was detected by immunohistochemistry analysis; and Fig.S3 Migration of MGC-803.SHIP2 cells and their corresponding counterparts was measured by wound-healing assays. (PDF 465 kb)
Rights and permissions
About this article
Cite this article
Ye, Y., Ge, Y.M., Xiao, M.M. et al. Suppression of SHIP2 contributes to tumorigenesis and proliferation of gastric cancer cells via activation of Akt. J Gastroenterol 51, 230–240 (2016). https://doi.org/10.1007/s00535-015-1101-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1101-0