Abstract
Background
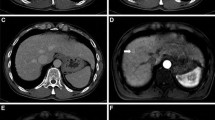
Gadoxetic acid-enhanced magnetic resonance imaging (MRI) has an important role in preoperative evaluation of hepatocellular carcinoma (HCC). However, no studies have prospectively performed intraindividual comparison of gadoxetic acid-enhanced 3T MRI and multidetector-row computed tomography (MDCT) with histopathological examination for the detection of HCCs. We prospectively compared the efficacies of gadoxetic acid-enhanced MRI and multiphasic contrast-enhanced MDCT with that of histopathological examination, used as a reference standard, for the detection of HCC in surgical candidates.
Methods
The study was approved by the institutional review boards at each of four centers. Patients scheduled to undergo multiphasic CT, gadoxetic acid-enhanced MRI, and liver surgery were prospectively included in this study. The diagnostic abilities of MRI and CT were evaluated and compared on the basis of sensitivity and positive predictive value for detection of and differentiation between HCCs and benign lesions.
Results
Fifty-four patients with 83 histopathologically confirmed HCCs were included in the study. Combined interpretation of the dynamic and hepatobiliary phases of gadoxetic acid-enhanced MRI showed statistically higher sensitivity for lesion detection (83 %) than did interpretation of multiphasic MDCT images (70 %; p < 0.001). The mean area under each alternative free-response receiver operating characteristics curve was significantly higher for MR images (0.927) than for CT images (0.864, p < 0.01).
Conclusions
The sensitivity for preoperative detection of HCCs was higher for gadoxetic acid-enhanced MRI than for multiphasic MDCT imaging.



Similar content being viewed by others
References
Kim SH, Choi D, Kim SH, et al. Ferucarbotran-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR. 2005;184:1069–76.
Kopp AF, Heuschmid M, Claussen CD. Multidetector helical CT of the liver for tumor detection and characterization. Eur Radiol. 2002;12:745–52.
Iannaccone R, Laghi A, Catalano C, et al. Hepatocellular carcinoma: role of unenhanced and delayed phase multi-detector row helical CT in patients with cirrhosis. Radiology. 2005;234:460–7.
Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104.
Peterson MS, Baron RL, Marsh JW Jr, Oliver JH 3rd, Confer SR, Hunt LE. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT-based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology. 2000;217:743–9.
Lim JH, Kim CK, Lee WJ, et al. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR. 2000;175:693–8.
Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445–54.
Krinsky GA, Lee VS, Theise ND, et al. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8:1156–64.
Rode A, Bancel B, Douek P, et al. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327–36.
Petersein J, Spinazzi A, Giovagnoni A, et al. Focal liver lesions: evaluation of the efficacy of gadobenate dimeglumine in MR imaging—a multicenter phase III clinical study. Radiology. 2000;215:727–36.
Kim JI, Lee JM, Choi JY, et al. The value of gadobenate dimeglumine-enhanced delayed phase MR imaging for characterization of hepatocellular nodules in the cirrhotic liver. Invest Radiol. 2008;43:202–10.
Kim YK, Kim CS, Chung GH, et al. Comparison of gadobenate dimeglumine-enhanced dynamic MRI and 16-MDCT for the detection of hepatocellular carcinoma. AJR. 2006;186:149–57.
Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD Initiative. Radiology. 2003;226:24–8.
Jang HJ, Lim JH, Lee SJ, Park CK, Park HS. DoYS. Hepatocellular carcinoma: are combined CT during arterial portography and CT hepatic arteriography in addition to triple-phase helical CT all necessary for preoperative evaluation? Radiology. 2000;215:373–80.
Kang BK, Lim JH, Kim SH, et al. Preoperative depiction of hepatocellular carcinoma: ferumoxides- enhanced MR imaging versus triple-phase helical CT. Radiology. 2003;226:79–85.
Huppertz A, Haraida S, Kraus A, et al. Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT—initial observations. Radiology. 2005;234:468–78.
Kim SH, Kim SH, Lee J, et al. Gadoxetic acid-enhanced MRI versus triplephase MDCT for the preoperative detection of hepatocellular carcinoma. AJR. 2009;192:1675–81.
Murakami T, Kim T, Takamura M, et al. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology. 2001;218:763–7.
Kwak HS, Lee JM, Kim CS. Preoperative detection of hepatocellular carcinoma: comparison of combined contrast-enhanced MR imaging and combined CT during arterial portography and CT hepatic arteriography. Eur Radiol. 2004;14:447–57.
Kim YK, Kim CS, Han YM, et al. Detection of hepatocellular carcinoma: gadoxetic acid-enhanced 3-dimensional magnetic resonance imaging versus multi-detector row computed tomography. J Comput Assist Tomogr. 2009;33:844–50.
Hwang J, Kim SH, Lee MW, Lee JY. Small (≤2 cm) hepatocellular carcinoma in patients with chronic liver disease: comparison of gadoxetic acid-enhanced 3.0 T MRI and multiphasic 64-multirow detector CT. Br J Radiol. 2012;85:e314–22.
Onishi H, Kim T, Imai Y, et al. Hypervascular hepatocellular carcinomas: detection with gadoxetate disodium-enhanced MR imaging and multiphasic multidetector CT. Eur Radiol. 2012;22:845–54.
Merkle EM, Dale BM, Paulson EK. Abdominal MR Imaging at 3.0 T. Magn Reson Imaging Clin N Am. 2006;14:17–26.
Ramalho M, Altun E, Heredia V, Zapparoli M, Semelka R. Liver MR imaging: 1.5 T versus 3 T. Magn Reson Imaging Clin N Am. 2007;15:321–47.
Hussain SM, Wielopolski PA, Martin DR. Abdominal magnetic resonance imaging at 3.0 T: problem or a promise for the future? Top Magn Reson Imaging. 2005;16:325–35.
von Falkenhausen MM, Lutterbey G, Morakkabati-Spitz N, et al. High-field-strength MR imaging of the liver at 3.0 T: intraindividual comparative study with MR imaging at 1.5 T. Radiology. 2006;241:156–66.
Tsurusaki M, Semelka RC, Zapparoli M, Elias J Jr, Altun E, Pamuklar E, Sugimura K. Quantitative and qualitative comparison of 3.0 T and 1.5 T MR imaging of the liver in patients with diffuse parenchymal liver disease. Eur J Radiol. 2009;72:314–20.
Ramalho M, Herédia V, Tsurusaki M, Altun E, Semelka RC. Quantitative and qualitative comparison of 1.5 and 3.0 Tesla MRI in patients with chronic liver diseases. J Magn Reson Imaging. 2009;29:869–79.
Zapparoli M, Semelka RC, Altun E, Tsurusaki M, Pamuklar E, Dale BM, Gasparetto EL, Elias J Jr. 3.0-T MRI evaluation of patients with chronic liver diseases: initial observations. Magn Reson Imaging. 2008;26:650–60.
Chang KJ, Kamel IR, Macura KJ, Bluemke DA. 3.0-T MR imaging of the abdomen: comparison with 1.5 T. Radiographics. 2008;28:1983–98.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsurusaki, M., Sofue, K., Isoda, H. et al. Comparison of gadoxetic acid-enhanced magnetic resonance imaging and contrast-enhanced computed tomography with histopathological examinations for the identification of hepatocellular carcinoma: a multicenter phase III study. J Gastroenterol 51, 71–79 (2016). https://doi.org/10.1007/s00535-015-1097-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1097-5