Abstract
Background
The levels of serum HER2 extracellular domain (ECD) are highly correlated with tissue HER2 status in metastatic gastric cancer (AGC). We sought to explore whether serum HER2 ECD could predict the efficacy of trastuzumab-treated HER2-positive AGC.
Materials and methods
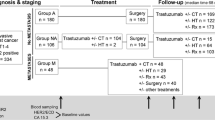
From 2011 to 2013, trastuzumab-treated AGC patients were enrolled. Serum HER2 ECD was centrally measured by chemiluminescence immunoassays (CLIA) method in available samples at baseline and after two cycles of trastuzumab combined with chemotherapy. The correlation between serum HER2 ECD and overall response rate (ORR) and progression-free survival (PFS) was analyzed.
Results
Sixty-five patients were analyzed with a median age of 58 years (range, 27–80 years). A significant difference of serum HER2 ECD levels was found between patients with HER2 IHC 3+ and those with HER2 IHC 2+ and FISH positive (p = 0.014). There was a significantly better ORR (80.00 vs. 50.00 %, p = 0.017) and PFS (median PFS, 268 vs. 139 days, p = 0.039) for patients with abnormal baseline serum HER2 ECD than for patients with normal serum HER2 ECD. Change in serum HER2 ECD during chemotherapy was significantly correlated with response to chemotherapy (79.17 vs. 51.52 %, p = 0.033) and PFS (median PFS, 303 vs. 147 days, p = 0.005) in patients with HER2-positive tumor tissue.
Conclusions
Our results support the clinical utility of measuring serum HER2 ECD levels in patients with advanced gastric cancer. Baseline and early changes in serum HER2 ECD could be useful for monitoring clinical outcome in HER2-positive AGC patients receiving trastuzumab-combined chemotherapy.




Similar content being viewed by others
References
Yan M, Parker BA, Schwab R, Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat Rev. 2014;40(6):770–80.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Boku N. HER2-positive gastric cancer. Gastric Cancer: Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2014;17(1):1–12.
Won E, Janjigian YJ, Ilson DH. HER2 directed therapy for gastric/esophageal cancers. Curr Treat Options Oncol. 2014;15(3):395–404.
Gomez-Martin C, Lopez-Rios F, Aparicio J, Barriuso J, Garcia-Carbonero R, Pazo R, et al. A critical review of HER2-positive gastric cancer evaluation and treatment: from trastuzumab, and beyond. Cancer Lett. 2014;351(1):30–40.
Lam L, McAndrew N, Yee M, Fu T, Tchou JC, Zhang H. Challenges in the clinical utility of the serum test for HER2 ECD. Biochim Biophys Acta. 2012;1826(1):199–208.
Carlson RW, Moench SJ, Hammond ME, Perez EA, Burstein HJ, Allred DC, et al. HER2 testing in breast cancer: NCCN task force report and recommendations. J Natl Compr Cancer Netw: JNCCN. 2006;4(Suppl 3:):S1–22 (quiz S3-4).
Leyland-Jones B, Smith BR. Serum HER2 testing in patients with HER2-positive breast cancer: the death knell tolls. Lancet Oncol. 2011;12(3):286–95.
Oyama K, Fushida S, Tsukada T, Kinoshita J, Watanabe T, Shoji M, et al. Evaluation of serum HER2-ECD levels in patients with gastric cancer. J Gastroenterol. 2015;50(1):121–2.
Dai SQ, An X, Wang F, Shao Q, Chen YC, Kong YN, et al. Serum HER 2 extracellular domain level is correlated with tissue HER 2 status in metastatic gastric or gastro-oesophageal junction adenocarcinoma. PLoS One. 2013;8(5):e63458.
Peng Z, Liu Y, Li Y, Zhang X, Zhou J, Lu M, et al. Serum HER2 extracellular domain as a potential alternative for tissue HER2 status in metastatic gastric cancer patients. Biomark Med. 2014;8(5):663–70.
Narita T, Seshimo A, Suzuki M, Murata J, Kameoka S. Status of tissue expression and serum levels of HER2 in gastric cancer patients in Japan. Hepatogastroenterology. 2013;60(125):1083–8.
Tagliabue E, Campiglio M, Pupa SM, Menard S, Balsari A. Activity and resistance of trastuzumab according to different clinical settings. Cancer Treat Rev. 2012;38(3):212–7.
Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (Herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61(12):4744–9.
Kim HA, Lee JK, Kim EK, Seol H, Noh WC. Serum human epidermal growth factor receptor 2 levels as a real-time marker for tumor burden in breast cancer patients. J Surg Oncol. 2014;109(5):421–5.
Witzel I, Loibl S, von Minckwitz G, Mundhenke C, Huober J, Hanusch C, et al. Monitoring serum HER2 levels during neoadjuvant trastuzumab treatment within the GeparQuattro trial. Breast Cancer Res Treat. 2010;123(2):437–45.
Petersen ER, Sorensen PD, Jakobsen EH, Madsen JS, Brandslund I. Serum HER-2 predicts response and resistance to trastuzumab treatment in breast cancer. Clin Chem Lab Med: CCLM/FESCC. 2013;51(7):1483–92.
Fornier MN, Seidman AD, Schwartz MK, Ghani F, Thiel R, Norton L, et al. Serum HER2 extracellular domain in metastatic breast cancer patients treated with weekly trastuzumab and paclitaxel: association with HER2 status by immunohistochemistry and fluorescence in situ hybridization and with response rate. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2005;16(2):234–9.
Ali SM, Carney WP, Esteva FJ, Fornier M, Harris L, Kostler WJ, et al. Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer. 2008;113(6):1294–301.
Gomez-Martin C, Plaza JC, Pazo-Cid R, Salud A, Pons F, Fonseca P, et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31(35):4445–52.
Acknowledgments
We thank the patients who participated in this study. The authors would also like to thank the staff of Translational Medicine Center, Laboratory of Oncology, Affiliated Hospital of Academy of Military Medical Sciences. This work was supported by National Natural Science Foundation of China (No. 81172110, 81472789), National High Technology Research and Development Program (No. 2012AA 02A 504), National Basic Research Program of China (No. 2014CBA02002), Beijing Natural Science Foundation (7142034) and China Postdoctoral Science Foundation (2013M530494). No writing assistance was utilized in the production of this manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Zhou and Z. Peng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, J., Peng, Z., Liu, Y. et al. Predictive value of serum HER2 ECD in patients with HER2-positive advanced gastric cancer treated with trastuzumab plus chemotherapy. J Gastroenterol 50, 955–961 (2015). https://doi.org/10.1007/s00535-015-1046-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-015-1046-3