Abstract
Background
Anti-TNF-α therapies interact with the tolerogenic response in patients with Crohn’s disease, modulating inflammation. However, drug levels and the genetic background may affect this interaction.
Methods
Patients with Crohn’s disease in remission on biologic monotherapy were enrolled in this study. FoxP3+ lymphocytes, NOD2 genotype, serum cytokine, anti-TNF-α levels, and anti-drug antibodies were evaluated. Regulatory T cell response to infliximab was evaluated in vitro.
Results
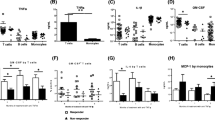
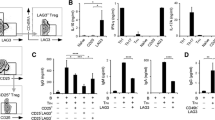
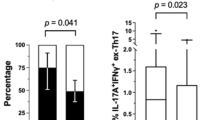
Fifty-seven patients were included. Thirty-nine patients (68.4 %) were receiving non-intensified biologic therapy whereas 18 patients (31.6 %) were under an intensified biologic schedule due to loss of response. Eleven intensified patients (61.1 %) showed a variant NOD2 genotype vs 9 on non-intensified biologics (23 %, p < 0.01). Percentage of FoxP3+ T cells and serum free anti-TNF-α levels were significantly higher in patients with a wild-type vs variant NOD2 genotype, either under non-intensified or intensified schedule. Increasing amounts of infliximab significantly increased the expression of FoxP3+ T cells and anti-TNF-α levels in the supernatant from wild-type NOD2 patients cultured cells whereas the induction of FoxP3+ T cells and anti-TNF-α levels in the supernatant from variant NOD2 patients cultured cells were significantly lower. TNF-α and IL-10 showed a correlation with the FoxP3+ T cell population percentage and serum levels of anti-TNF-α, irrespective of NOD2 genotype. Eight variant NOD2 patients (66.6 %) vs 4 wild-type NOD2 patients (8.8 %) showed a perianal phenotype (p = 0.01). A significant reduction of the percentage of FoxP3+ T cells and serum levels of anti-TNF-α was observed in patients with the associated perianal disease.
Conclusion
Anti-TNF-α loss of response is associated with a decreased percentage of FoxP3+ T cells and a variant NOD2 genotype in patients with CD.





Similar content being viewed by others
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29.
Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S.
Obermeier F, Dunger N, Strauch UG, et al. CpG motifs of bacterial DNA essentially contribute to the perpetuation of chronic intestinal inflammation. Gastroenterology. 2005;129:913–27.
Elson CO, Cong Y, McCracken VJ, et al. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76.
Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118–25.
Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–53.
Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–6.
Gutierrez A, Frances R, Amoros A, et al. Cytokine association with bacterial DNA in serum of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:508–14.
Gutierrez A, Holler E, Zapater P, et al. Antimicrobial peptide response to blood translocation of bacterial DNA in Crohn’s disease is affected by NOD2/CARD15 genotype. Inflamm Bowel Dis. 2011;17:1641–50.
Roncarolo MG, Gregori S, Battaglia M, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50.
Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500.
Danese S, Fiorino G, Rutella S. Regulatory T-cell therapy for Crohn’s disease: in vivo veritas. Gastroenterology. 2012;143:1135–8.
Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302.
Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+ CD25+ regulatory T cells. J Immunol. 2003;170:3939–43.
Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+ CD4+ CD25+ and IL-10-secreting CD4+ CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–60.
Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78.
Desreumaux P, Foussat A, Allez M, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143(1207–1217):e1201–2.
Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-alpha therapy. Aliment Pharmacol Ther. 2011;34:1–10.
Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–95.
Dassopoulos T, Sninsky CA. Optimizing immunomodulators and anti-TNF agents in the therapy of Crohn disease. Gastroenterol Clin North Am. 2012;41:393–409.
Li Z, Arijs I, De Hertogh G, et al. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16:1299–310.
Boschetti G, Nancey S, Sardi F, et al. Therapy with anti-TNFalpha antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:160–70.
Di Sabatino A, Biancheri P, Piconese S, et al. Peripheral regulatory T cells and serum transforming growth factor-beta: relationship with clinical response to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2010;16:1891–7.
Guidi L, Felice C, Procoli A, et al. FOXP3(+) T regulatory cell modifications in inflammatory bowel disease patients treated with anti-TNFalpha agents. Bio Med Res Int. 2013;2013:286368.
Grundstrom J, Linton L, Thunberg S, et al. Altered immunoregulatory profile during anti-tumour necrosis factor treatment of patients with inflammatory bowel disease. Clin Exp Immunol. 2012;169:137–47.
Veltkamp C, Anstaett M, Wahl K, et al. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFalpha treatment. Gut. 2011;60:1345–53.
Bell MP, Svingen PA, Rahman MK, et al. FOXP3 regulates TLR10 expression in human T regulatory cells. J Immunol. 2007;179:1893–900.
Rahman MK, Midtling EH, Svingen PA, et al. The pathogen recognition receptor NOD2 regulates human FOXP3+ T cell survival. J Immunol. 2010;184:7247–56.
Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–32.
Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5–36.
Hampe J, Grebe J, Nikolaus S, et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn’s disease: a cohort study. Lancet. 2002;359:1661–5.
Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8.
Rutgeerts P, Van AG, Vermeire S. Review article: infliximab therapy for inflammatory bowel disease—seven years on. Aliment Pharmacol Ther. 2006;23:451–63.
Candon S, Mosca A, Ruemmele F, et al. Clinical and biological consequences of immunization to infliximab in pediatric Crohn’s disease. Clin Immunol. 2006;118:11–9.
Gutierrez A, Scharl M, Sempere L, et al. Genetic susceptibility to increased bacterial translocation influences the response to biological therapy in patients with Crohn’s disease. Gut. 2014;63:272–80.
Long SH, He Y, Chen MH, et al. Activation of PI3K/Akt/mTOR signaling pathway triggered by PTEN downregulation in the pathogenesis of Crohn’s disease. J Dig Dis. 2013;14:662–9.
Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83–9.
Sandborn WJ, Fazio VW, Feagan BG, et al. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–30.
Galandiuk S, Kimberling J, Al-Mishlab TG, et al. Perianal Crohn disease: predictors of need for permanent diversion. Ann Surg. 2005;241:796–801 (discussion 801–792).
Crawford NP, Colliver DW, Eichenberger MR, et al. CARD15 genotype–phenotype relationships in a small inflammatory bowel disease population with severe disease affection status. Dig Dis Sci. 2007;52:2716–24.
Alvarez-Lobos M, Arostegui JI, Sans M, et al. Crohn’s disease patients carrying Nod2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Ann Surg. 2005;242:693–700.
Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–9.
Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–53.
Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402–13.
Ehrenstein MR, Evans JG, Singh A, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85.
Saruta M, Yu QT, Fleshner PR, et al. Characterization of FOXP3+ CD4+ regulatory T cells in Crohn’s disease. Clin immunol. 2007;125:281–90.
Acknowledgments
This work has been partially supported by Grant PI13/1443 from the Instituto de Salud Carlos III, Madrid, Spain.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Juanola, O., Moratalla, A., Gutiérrez, A. et al. Anti-TNF-alpha loss of response is associated with a decreased percentage of FoxP3+ T cells and a variant NOD2 genotype in patients with Crohn’s disease. J Gastroenterol 50, 758–768 (2015). https://doi.org/10.1007/s00535-014-1020-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-1020-5