Abstract
Background
Triple therapy with telaprevir (TVR), pegylated interferon and ribavirin has improved antiviral efficacy in patients with chronic hepatitis C (CH-C). However, the severe adverse effects caused by TVR are important to resolve. In this prospective, randomized, multicenter, open-label study, the antiviral efficacy and safety in the reduced administration of TVR were examined.
Methods
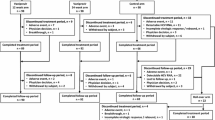
A total of 81 CH-C Japanese patients with HCV genotype 1 were randomized into two regimens of TVR 2250 mg (TVR-2250) or 1500 mg (TVR-1500) and treated with triple therapy for 24 weeks.
Results
The mean HCV RNA at start, 2 and 4 weeks of treatment were 6.69 ± 0.70, 1.05 ± 0.74, 0.22 ± 0.48 log10 IU/ml in the TVR-2250 group and 6.70 ± 0.62, 1.02 ± 0.62, 0.13 ± 0.41 log10 IU/ml in the TVR-1500 group. The SVR rates were 85 % in both groups (35/41 and 34/40, respectively). There were no patients with viral breakthrough in either group. As for adverse effects, rash more than moderate and severe anemia with <8.5 g/dl of hemoglobin were higher in the TVR-2250 group than in the TVR-1500 group (p = 0.046, p < 0.001, respectively). The increase in serum creatinine levels and decrease in estimated glomerular filtration rates were higher in the TVR-2250 group than in the TVR-1500 group.
Conclusions
The lower dose of TVR (1500 mg/day) can result in similar SVR rates and lower treatment-related adverse effects compared to the higher dose of TVR (2250 mg/day) in triple therapy (UMIN: 000007313, 000007330).





Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C virus
- IFN:
-
Interferon
- Peg-IFN:
-
Pegylated interferon
- RBV:
-
Ribavirin
- PI:
-
Protease inhibitor
- TVR:
-
Telaprevir
- SVR:
-
Sustained virologic response
- EOT:
-
End of treatment
- HCC:
-
Hepatocellular carcinoma
- CH-C:
-
Chronic hepatitis C
- Hb:
-
Hemoglobin
- WBC:
-
White blood cell
- RVR:
-
Rapid virologic response
- c-EVR:
-
Complete early virologic response
- ETR:
-
End of treatment response
- SMV:
-
Simeprevir
References
Ghany MG, Nelson DR, Strader DB, et al. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–44.
McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827–38.
Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–50.
McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–303.
Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16.
Sherman KE, Flamm SL, Afdhal NH, et al. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–24.
Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–28.
Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78–84.
Hayashi N, Okanoue T, Tsubouchi H, Toyota J, Chayama K, Kumada H. Efficacy and safety of telaprevir, a new protease inhibitor, for difficult-to-treat patients with genotype 1 chronic hepatitis C. J Viral Hepat. 2012;19:134–42.
Hezode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC)—NCT01514890. J Hepatol. 2013;59:434–41.
Reesink HW, Zeuzem S, Weegink CJ, et al. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology. 2006;131:997–1002.
Suzuki F, Akuta N, Suzuki Y, et al. Rapid loss of hepatitis C virus genotype 1b from serum in patients receiving a triple treatment with telaprevir (MP-424), pegylated interferon and ribavirin for 12 weeks. Hepatol Res. 2009;39:1056–63.
Suzuki F, Suzuki Y, Sezaki H, et al. Exploratory study on telaprevir given every 8 h at 500 mg or 750 mg with peginterferon-alpha-2b and ribavirin in hepatitis C patients. Hepatol Res. 2013;43:691–701.
Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93.
Kawakami Y, Suzuki F, Karino Y, et al. Telaprevir is effective given every 12 hours at 750 mg with peginterferon-alfa-2b and ribavirin to Japanese patients with HCV-1b IL28B rs8099917 TT. Antivir Ther. 2013. doi:10.3851/IMP2706.
Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918–29.
Zeuzem S, Berg T, Gane E, et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology. 2014;146:430–41.
Hayashi N, Seto C, Kato M, et al. Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naive hepatitis C genotype 1-infected patients in Japan: the DRAGON study. J Gastroenterol. 2014;49:138–47.
Akuta N, Suzuki F, Hirakawa M, et al. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421–9.
Acknowledgments
Other institutions and participants in the Osaka Liver Forum are the following: National Hospital Organization Minami Wakayama Medical Center, M Kato; Osaka General Medical Center, A Inoue; Kinki Central Hospital of Mutual Aid Association of Public School Teachers, E Hayashi; Osaka Medical Center for Cancer and Cardiovascular Diseases, K Katayama; National Hospital Organization Osaka Minami Medical Center, T Hijioka; Osaka Koseinenkin Hospital, Y Ito; Yao Municipal Hospital, H Fukui; National Hospital Organization Osaka National Hospital, E Mita; Kansai Rousai Hospital, H Hagiwara; Higashiosaka City Central Hospital, S Iio,; Toyonaka Municipal Hospital, M Inada; Itami City Hospital, Y Saji; Otemae Hospital, Y Doi; Suita Municipal Hospital, T Nagase; Ashiya Municipal Hospital, A Takeda; Nishinomiya Municipal Central Hospital, H Ogawa; Kaizuka City Hospital, Y Yamada; Izumiotsu Municipal Hospital, S Yamagata; Osaka Kaisei Hospital, N Imaizumi; Kano General Hospital, S Kubota; Saso Hospital, M Nishiuchi; and Meiwa Hospital, Y Hayakawa.
This work was supported by a Grant-in-Aid for Research on Hepatitis and BSE from the Ministry of Health Labour and Welfare of Japan and a Scientific Research from the Ministry of Education, Science, and Culture of Japan.
Conflict of interest
Professor Tetsuo Takehara received scholarship funds from Merck Sharp & Dohme K.K. Co., Ltd. and Chugai Pharmaceutical Co., Ltd. Other authors declare thath they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oze, T., Hiramatsu, N., Yakushijin, T. et al. The prospective randomized study on telaprevir at 1500 or 2250 mg with pegylated interferon plus ribavirin in Japanese patients with HCV genotype 1. J Gastroenterol 50, 313–322 (2015). https://doi.org/10.1007/s00535-014-0965-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-0965-8