Abstract
Background
Esophageal squamous cell carcinoma (ESCC) is a common cancer type in China. In this study, we aimed to develop aneuploidy markers for diagnosis and prognosis of ESCC.
Methods
Chromosomal aneuploidies were detected in 493 primary tumors and 61 precancerous lesions by fluorescence in situ hybridization with chromosome enumeration probes (CEP), and cut-off values were set by receiver operating characteristic (ROC) curves.
Results
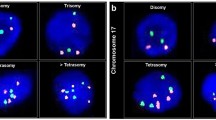
According to the cut-off values, chromosomes 3, 8, 10, 12, 17 and 20 presented frequent gains, with rates of 70.1, 69.7, 58.9, 66.9, 67.5 and 77.2 % in tumors and of 32.1, 26.8, 33.9, 41.2, 44.0 and 42.0 % in precancerous lesions. Loss of chromosome Y was detected in 72.0 % of male patients. An optimal four-probe panel CEP3/12/17/20 was established for detecting ESCC (sensitivity: 86.1 %), and CEP3/10/12/20 for precancerous lesions (sensitivity: 48.0 %). Gain of CEP8 was significantly correlated with lymph node metastasis (LNM) and late stages (P = 0.002 and 0.001), and loss of CEPY with age (P = 0.002, male). Kaplan–Meier survival curves indicated that patients with positive CEP10/17 (pT1 + T2, P = 0.041) and CEP8/17 (stages IIb + III + IV, P = 0.002) had poor overall survival. Combinations of LNM/stage and CEP panels could divide patients into more subgroups, including LNM + CEP3/17, LNM + CEP10/17, LNM + CEP3/10/17, stage + CEP3/17, stage + CEP10/17 and stage + CEP3/10/17 (P = 0.0004, 0.0003, 0.0001, 0.005, 0.001 and 0.0008, respectively). Multivariate Cox regression analysis confirmed that the above combinational models were independent prognostic factors.
Conclusions
Our data suggest that the combinational probe sets may have potential for detection and prognostic prediction of ESCC.



Similar content being viewed by others
Abbreviations
- ESCC:
-
Esophageal squamous cell carcinoma
- CEP:
-
Chromosome enumeration probes
- CGH:
-
Comparative genomic hybridization
- SNP:
-
Single nucleotide polymorphism
- DAPI:
-
40,6-diamidino-2-phenylindole
- PBS:
-
Phosphate-buffered saline
- FISH:
-
Fluorescence in situ hybridization
- CCD:
-
Cooled charged-coupled device
- OS:
-
Overall survival
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- HIN:
-
High-grade intra-epithelial neoplasia
- LIN:
-
Low-grade intra-epithelial neoplasia
- LNM:
-
Lymph node metastasis
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- CF:
-
Clinico-pathological features
References
Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533–43.
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–52.
Duesberg P, Li R, Rasnick D, et al. Aneuploidy precedes and segregates with chemical carcinogenesis. Cancer Genet Cytogenet. 2000;119(2):83–93.
Albertson DG, Collins C, McCormick F, et al. Chromosome aberrations in solid tumors. Nat Genet. 2003;34(4):369–76.
Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5(10):773–85.
Weaver BA, Silk AD, Montagna C, et al. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11(1):25–36.
Sotillo R, Hernando E, Diaz-Rodriguez E, et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11(1):9–23.
Stephens JK, Bibbo M, Dytch H, et al. Correlation between automated karyometric measurements of squamous cell carcinoma of the esophagus and histopathologic and clinical features. Cancer. 1989;64(1):83–7.
Ruol A, Segalin A, Panozzo M, et al. Flow cytometric DNA analysis of squamous cell carcinoma of the esophagus. Cancer. 1990;65(5):1185–8.
Sasaki K, Murakami T, Nakamura M. Intratumoral heterogeneity in DNA ploidy of esophageal squamous cell carcinomas. Cancer. 1991;68(11):2403–6.
Blant SA, Ballini JP, Caron CT, et al. Evolution of DNA ploidy during squamous cell carcinogenesis in the esophagus. Dis Esophagus. 2001;14(3–4):178–84.
Saeki H, Kimura Y, Ito S, et al. Biologic and clinical significance of squamous epithelial dysplasia of the esophagus. Surgery. 2002;131(1 Suppl):S22–7.
Mohan V, Ponnala S, Reddy HM, et al. Chromosome 11 aneusomy in esophageal cancers and precancerous lesions–an early event in neoplastic transformation: an interphase fluorescence in situ hybridization study from south India. World J Gastroenterol. 2007;13(4):503–8.
Sokolova IA, Halling KC, Jenkins RB, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn. 2000;2(3):116–23.
Kruger S, Mess F, Bohle A, et al. Numerical aberrations of chromosome 17 and the 9p21 locus are independent predictors of tumor recurrence in non-invasive transitional cell carcinoma of the urinary bladder. Int J Oncol. 2003;23(1):41–8.
Zellweger T, Benz G, Cathomas G, et al. Multi-target fluorescence in situ hybridization in bladder washings for prediction of recurrent bladder cancer. Int J Cancer. 2006;119(7):1660–5.
Dimashkieh H, Wolff DJ, Smith TM, et al. Evaluation of urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 2013;121(10):591–7.
Yen CC, Chen YJ, Chen JT, et al. Comparative genomic hybridization of esophageal squamous cell carcinoma: correlations between chromosomal aberrations and disease progression/prognosis. Cancer. 2001;92(11):2769–77.
Wei F, Ni J, Wu SS, et al. Cytogenetic studies of esophageal squamous cell carcinomas in the northern Chinese population by comparative genomic hybridization. Cancer Genet Cytogenet. 2002;138(1):38–43.
Noguchi T, Kimura Y, Takeno S, et al. Chromosomal imbalance in esophageal squamous cell carcinoma: 3q gain correlates with tumor progression but not prognostic significance. Oncol Rep. 2003;10(5):1393–400.
Kwong D, Lam A, Guan X, et al. Chromosomal aberrations in esophageal squamous cell carcinoma among Chinese: gain of 12p predicts poor prognosis after surgery. Hum Pathol. 2004;35(3):309–16.
Carneiro A, Isinger A, Karlsson A, et al. Prognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer. 2008;8:98.
Qin YR, Wang LD, Fan ZM, et al. Comparative genomic hybridization analysis of genetic aberrations associated with development of esophageal squamous cell carcinoma in Henan, China. World J Gastroenterol. 2008;14(12):1828–35.
Shi ZZ, Liang JW, Zhan T, et al. Genomic alterations with impact on survival in esophageal squamous cell carcinoma identified by array comparative genomic hybridization. Genes Chromosomes Cancer. 2011;50(7):518–26.
Bandla S, Pennathur A, Luketich JD, et al. Comparative genomics of esophageal adenocarcinoma and squamous cell carcinoma. Ann Thorac Surg. 2012;93(4):1101–6.
Chattopadhyay I, Singh A, Phukan R, et al. Genome-wide analysis of chromosomal alterations in patients with esophageal squamous cell carcinoma exposed to tobacco and betel quid from high-risk area in India. Mutat Res. 2010;696(2):130–8.
Hu N, Wang C, Ng D, et al. Genomic characterization of esophageal squamous cell carcinoma from a high-risk population in China. Cancer Res. 2009;69(14):5908–17.
Hu N, Wang C, Hu Y, et al. Genome-wide loss of heterozygosity and copy number alteration in esophageal squamous cell carcinoma using the Affymetrix GeneChip Mapping 10 K array. BMC Genom. 2006;7:299.
Arteaga CL, Sliwkowski MX, Osborne CK, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9(1):16–32.
Dent S, Oyan B, Honig A, et al. HER2-targeted therapy in breast cancer: a systematic review of neoadjuvant trials. Cancer Treat Rev. 2013;39(6):622–31.
Dressler LG, Berry DA, Broadwater G, et al. Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol. 2005;23(19):4287–97.
Skacel M, Fahmy M, Brainard JA, et al. Multitarget fluorescence in situ hybridization assay detects transitional cell carcinoma in the majority of patients with bladder cancer and atypical or negative urine cytology. J Urol. 2003;169(6):2101–5.
Varella-Garcia M, Akduman B, Sunpaweravong P, et al. The UroVysion fluorescence in situ hybridization assay is an effective tool for monitoring recurrence of bladder cancer. Urol Oncol. 2004;22(1):16–9.
Oki E, Hisamatsu Y, Ando K, et al. Clinical aspect and molecular mechanism of DNA aneuploidy in gastric cancers. J Gastroenterol. 2012;47(4):351–8.
Wang LD, Qin YR, Fan ZM, et al. Comparative genomic hybridization: comparison between esophageal squamous cell carcinoma and gastric cardia adenocarcinoma from a high-incidence area for both cancers in Henan, northern China. Dis Esophagus. 2006;19(6):459–67.
Hu N, Clifford RJ, Yang HH, et al. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genom. 2010;11:576.
Tada K, Oka M, Tangoku A, et al. Gains of 8q23-qter and 20q and loss of 11q22-qter in esophageal squamous cell carcinoma associated with lymph node metastasis. Cancer. 2000;88(2):268–73.
Ueno T, Tangoku A, Yoshino S, et al. Gain of 5p15 detected by comparative genomic hybridization as an independent marker of poor prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2002;8(2):526–33.
Wang Q, Zhu H, Xiao Z, et al. Expression of epidermal growth factor receptor is an independent prognostic factor for esophageal squamous cell carcinoma. World J Surg Oncol. 2013;11(1):278.
Zhang YF, Xu QX, Liao LD, et al. kappa-Opioid receptor in the nucleus is a novel prognostic factor of esophageal squamous cell carcinoma. Hum Pathol. 2013;44(9):1756–65.
Shimizu H, Shiozaki A, Ichikawa D, et al. The expression and role of Aquaporin 5 in esophageal squamous cell carcinoma. J Gastroenterol. 2014;49(4):655-66.
Shiozaki A, Iitaka D, Ichikawa D, et al. xCT, component of cysteine/glutamate transporter, as an independent prognostic factor in human esophageal squamous cell carcinoma. J Gastroenterol. 2013.
Hsu PK, Chen HY, Yeh YC, et al. TPX2 expression is associated with cell proliferation and patient outcome in esophageal squamous cell carcinoma. J Gastroenterol. 2013.
Acknowledgments
The authors thank Professors Shu-Jun Cheng and Yan-Ning Gao for providing CEP clones. This work was supported by the National High-Tech R&D Program of China (2012AA02A503, 2012AA02A209) and the National Natural Science Foundation of China (81330052, 81321091).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Hao and H. Yao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2014_961_MOESM2_ESM.eps
Supplementary Fig. 1 Prognostic impact of combinations of clinico-pathological parameters and CEP probes on ESCC patients with therapy after surgery. (a) LNM, (b) LNM + CEP3, (c) LNM + CEP8, (d) LNM + CEP10, (e) LNM + CEP17, (f) stage, (g) stage + CEP8, (h) LNM + CEP3/17, (i) LNM + CEP10/17, (j) LNM + CEP3/10/17. (k) stage + CEP3/17, (l) stage + CEP10/17, (m) stage + CEP3/10/17. “+” and “-” represent gains and no gains, respectively (EPS 2205 kb)
535_2014_961_MOESM3_ESM.eps
Supplementary Fig. 2 Prognostic impact of combinations of clinico-pathological parameters and CEP probes on ESCC patients without therapy after surgery. (a) LNM, (b) LNM + CEP3, (c) LNM + CEP8, (d) LNM + CEP10, (e) LNM + CEP17, (f) stage, (g) stage + CEP8, (h) LNM + CEP3/17, (i) LNM + CEP10/17, (j) LNM + CEP3/10/17. (k) stage + CEP3/17, (l) stage + CEP10/17, (m) stage + CEP3/10/17. “+” and “-” represent gains and no gains, respectively 3 (EPS 2233 kb)
Rights and permissions
About this article
Cite this article
Hao, JJ., Yao, HQ., Dai, GY. et al. Chromosomal aneuploidies and combinational fluorescence in situ hybridization probe panels are useful for predicting prognosis for esophageal squamous cell carcinoma. J Gastroenterol 50, 155–166 (2015). https://doi.org/10.1007/s00535-014-0961-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-0961-z