Abstract
Intrahepatic cholangiocarcinoma is an aggressive malignancy and is one of the most devastating cancers of the gastrointestinal tract. The molecular mechanisms contributing to the pathogenesis of these cancers are not well understood. The recognition and distinction of these cancers from other tumors such as perihilar or extrahepatic distal cholangiocarcinoma and hepatocellular carcinoma are important in defining the pathogenesis. New insights into molecular mechanisms contributing to disease pathogenesis are emerging from recent epidemiological, genome-wide profiling and laboratory based studies. These have contributed to an improved understanding of risk factors, genetic mutations and pathophysiological mechanisms that are associated with these tumors. The contribution of well-established risk factors such as biliary tract inflammation and key signaling pathways involved in intrahepatic cholangiocarcinoma are being further defined. These new insights have several important implications for both molecular diagnosis and therapy of these cancers.
Similar content being viewed by others
Introduction
Cholangiocarcinomas are primary malignancies of the biliary tract [1]. As a group, these malignancies are aggressive and are associated with a very poor prognosis. This review will focus on the molecular pathogenesis of intrahepatic cholangiocarcinoma (iCCA). The incidence and mortality rates of these cancers are increasing worldwide [2–5]. In contrast to most other tumors of the liver and gastrointestinal tract, such as hepatocellular, gastric or pancreatic cancers, the pathogenesis of these tumors is very poorly understood [6]. There are several diverse risk factors associated with these cancers, but the pathogenetic mechanisms associated with these risk factors have not been elucidated. Recently, there have been several observations that provide additional insights into the pathogenesis of iCCA. These findings stem from many different sources such as epidemiological studies, genome wide profiling studies and laboratory based studies. In this review, we discuss some of these recent findings and highlight how these may lead to more effective management of these cancers.
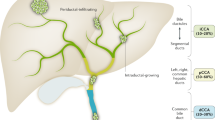
Nomenclature of biliary tract cancers
Cancers of the biliary tract can arise from several distinct sites [1]. These include the gall-bladder, intrahepatic ducts, large extrahepatic ductal system, and the perihilar region. While cancers of the gall bladder and ampulla are easily distinguished and usually considered as distinct tumor types, cancers of the intrahepatic and extrahepatic biliary tract have been considered together as cholangiocarcinoma. Differences in clinical presentation and treatment outcomes of the different cancer types that are referred to as cholangiocarcinoma are well recognized. However, the distinct types of cancer have not been adequately delineated in epidemiological classifications, clinical practice, or basic research studies. Differences in risk factors, histological features and genetic changes can be recognized between intrahepatic, hilar or ductal cholangiocarcinomas [7–9]. Because of these clinical and biological differences, interpreting data that is derived from studies where these entities are considered together is problematic. Lack of a clear distinction between these cancers has been a major limitation in identifying specific risk factors and understanding disease pathogenesis.
A convention that is accepted in clinical practice and appropriate for use in population based studies or clinical trials makes a distinction between iCCA, tumors arising from the small bile ducts in the liver, and perihilar cholangiocarcinoma (pCCA) or distal extrahepatic cholangiocarcinoma (dCCA), arising from the large ductal system at the hilum or outside the liver [10, 11]. Although there are clear differences in risk factors for intrahepatic and extrahepatic tumors, gene expression profiling studies have not revealed evident differences in genetic profiles between these. Hilar tumors warrant separate consideration because of their unique location, presentation and management considerations, and are more similar to distal extrahepatic cancers than to iCCA. Future studies in rigorously defined groups of these tumors are needed in order to define their specific risk factors and pathogenesis.
Classifications of intrahepatic cholangiocarcinoma
The iCCA are macroscopically and microscopically diverse tumors. Several classifications have been described for iCCA, on the basis of site of origin, extent of disease, gross morphology, histology or postulated cell type of origin. The macroscopic classification of the Liver Cancer Study Group of Japan recognizes a mass-forming, and a mixed mass-forming and periductal infiltrating type which are the most frequently encountered types [12]. Less commonly, periductal infiltrating or intraductal tumors can occur without mass formation. Most peripheral small duct iCCA are of the mass-forming type, whereas those that develop within the large second order intrahepatic bile ducts can be of the periductal infiltrating, mixed or intraductal growth subtypes.
Histologically, the majority of iCCA are well-to-moderately differentiated adenocarcinomas with varying degrees of desmoplasia [13]. A recent classification proposed by Nakanuma et al. [14] divides iCCA into conventional type, bile ductular type, intraductal neoplasms type and rare variants such as combined HCC-CCA, and undifferentiated iCCA. This classification is based on gross morphology and pathological similarities between biliary and pancreatic neoplasms, but also incorporates hepatic progenitor cell/stem cell phenotype and is compatible with other proposed classifications based on cell lineage [2, 15]. Although these classifications may be helpful in defining pathological categories of iCCA, the clinical utility of classifications that are based on type of cell or histology are not yet established.
New insights from epidemiological studies
The commonly recognized risk factors of biliary tract cancers, including iCCA, comprise a diverse group of conditions that include infectious diseases, congenital conditions, inflammatory diseases, and drugs and toxins [6, 16–20]. Some of these factors may not be relevant for iCCA. Indeed, many cases of iCCA arise sporadically and are not associated with any of these risk factors. Most reports of risk factors for biliary cancers do not distinguish between iCCA and other types of cholangiocarcinomas such as pCCA or dCCA. Recent epidemiological studies have identified new occupational and environmental risk factors for iCCA. In a recent report, several cases of cholangiocarcinoma were identified amongst workers at a printing company in Osaka [21]. Subsequently, cases were identified in workers in other printing works in Japan. The increased risk was postulated to arise from exposure to inhaled volatile organic compounds such as 1,2, dichloropropane and dichloromethane, that were used to wash off ink from printing machines [21].
Another environmental exposure that has recently been recognized is asbestos. The association between iCCA and asbestos, smoking history, and socio-occupational status was examined in a recent study using conditional logistic regression models. Occupational exposure to asbestos appeared to be strongly associated with iCCA, but not with other types of cholangiocarcinomas [22].
The presence of chronic viral hepatitis and liver cirrhosis have also emerged as a risk factor for iCCA in several recent studies [16, 18, 23–29]. In a recent meta-analysis, we examined the evidence for cirrhosis as a risk factor from published, well described case–control studies [30]. This analysis showed an odds ratio of 22.9 for cirrhosis as a risk factor for iCCA [30]. In addition, a comprehensive meta-analysis of several other risk factors for iCCA supported an association of chronic hepatitis B, chronic hepatitis C and alcohol with iCCA. These are all risk factors for cirrhosis and could, therefore, explain the increased risk of iCCA with cirrhosis (Fig. 1). The risk associated with smoking, diabetes and obesity were less apparent in this meta-analysis. These observations from epidemiological studies have identified several previously unrecognized risk factors for iCCA that include cirrhosis, hepatitis B, hepatitis C, obesity, diabetes, alcohol and possibly also asbestos and some organic solvents.
New insights from genomic profiling studies
The molecular pathogenesis of iCCA may have similarities with the pathogenesis of hepatocellular cancer because many of the dominant risk factors associated with HCC such as cirrhosis, HBV, HCV and metabolic syndrome are also risk factors for iCCA. This is further supported by the results of recent transcriptome analysis studies that have identified common genomic traits between these cancers [31–33]. Gene signatures of a poorly prognostic group of iCCA are similar to those observed in poor-prognosis HCC and with stem-like molecular signatures [34, 35]. Based on these, it is postulated that these distinct subgroups of iCCA and HCC may share a common stem cell origin. Although the prevalence of iCCA derived from a stem cell population is unknown, up to 25 % of HCCs may be derived from stem cells [36]. Differences in cellular origin and pathogenesis could account for epidemiological or clinical heterogeneity of these tumors. Based on genomic profiling, molecular analysis can identify clinically distinct classes of iCCA [37]. However, further studies are needed to determine the utility of molecular profiling based classifications for iCCA.
The tumor stroma and local microenvironment play a crucial role in cancer progression and in therapeutic responses in many different cancers. A contribution of the stromal compartment to the pathogenesis of iCCA has also been recognized [31, 38]. Examination of tumor cell and stromal gene expression in a large series of surgically resected cholangiocarcinomas showed that the most malignant tumor phenotype was characterized by changes in epithelial cells (e.g., upregulation of HER-2 signaling) as well as in stromal cells (e.g., overexpression of proinflammatory cytokines IL-6 and CXCR4). A 238 gene-group was identified that correlated with a high risk group and overall or recurrence-free survival. This group could be further reduced to 36 genes, many of which were cholangio-specific. Moreover, these genes could predict outcomes in HCC and other tumors. A 26-gene stromal derived prognostic predictor gene signature that was identified in breast cancer was also noted to be enriched in the stromal compartment of CCA. These genomic studies identify gene changes in both tumor cells and stromal cells that are associated with CCA growth and spread.
Recent data from whole transcriptome sequencing has identified the presence of gene fusions that are associated with iCCA such as FGFR2-BICC and FIG-ROS [39]. These may result in sensitivity to kinase inhibition, and represent a novel area of insight into subgroups that may be responsive to targeted therapy. To date there are few reported studies of deep sequencing in well-defined cohorts of iCCA, and such studies are likely to provide further insights into genetic events associated with the pathogenesis of iCCA.
New insights into the origin of ICC from laboratory based investigations
Recent studies have implicated Notch signaling in the pathogenesis of cholangiocarcinoma. Notch signaling is an evolutionary conserved pathway that contributes to cell differentiation and maintenance of tissue homeostasis. Notch is involved in biliary tract differentiation, and disrupted Notch signaling results in congenital hypoplasia of the biliary tract. Over-expression of the intercellular domain of Notch results in liver tumor formation with features of cholangiocarcinoma [40]. Moreover, activated AKT and NICD result in rapid induction of liver tumors [41]. Furthermore, thioacetamide derived tumor formation is enhanced in the presence of activated Notch [42].
These studies also provide new insights into potential cells of origin for iCCA. It has been presumed that iCCA arises from the malignant transformation of cholangiocytes, although direct experimental evidence for this has been lacking. In a subset of cases, tumors may arise from multipotent stem cell populations such as hepatic progenitor cells that undergo transformation while undergoing maturation with cholangiocytic differentiation. Hepatic progenitor cells and cells within the glands of the bile duct are putative candidates for such stem cells. Within the liver, progenitor cells are located in the most peripheral branches of the biliary tree, ductules and canal of Hering. These cells can differentiate into either hepatocytes or cholangiocytes. Tumors with mixed phenotypes and varying hepatocellular and cholangiocellular differentiation characteristics could result when a progenitor cell that is not fully differentiated develops into a cancer following maturational arrest. Cells within the peribiliary glands could also give rise to tumors with a biliary phenotype and may constitute the cell of origin for a subset of iCCA as well as dCCA and pCCA.
In addition to stem or progenitor populations, fully differentiated mature hepatocytes could serve as a cell of origin of iCCA based on studies using cell lineage tracing studies [41, 42]. Sekiya and Suzuki showed that Notch mediated conversion of hepatocytes can result in iCCA, using a tamoxifen inducible Cre/lox mouse model system that allowed lineage studies of hepatocytes (expressing Alb) or cholangiocytes (expressing CK19). Mice received thioacetamide, which induced tumor nodules with a tubular phenotype. The frequency of tumor formation was increased when mice received activated Notch 1, and was reduced when mice received an inhibitor of Notch (Hes 1). In a different study, Fan et al., showed that injection of the intracellular domain of Notch 1 with the Akt overexpressing plasmid resulted in rapid induction of iCCA that arise from hepatocytes in mice treated with thioacetamide. Interestingly, the origin of these was not in the periportal areas where the hepatic stem cell/progenitor niche is located, but in the central areas of the liver lobule. Although the cell types of origin of iCCA in humans are still unknown, these observations suggest that tumors could arise from several different types of cells such as differentiated hepatocytes, dysplastic or immature cholangiocytes, or from multipotent cell niches such as hepatic stem/progenitor cells, or from within peribiliary glands.
Molecular pathogenesis
Key molecular events involved in the pathogenesis of iCCA are summarized in Fig. 2. In the classical model of tumor pathogenesis, promotion of tumor development follows chronic biliary inflammation with the release of inflammatory mediators, and occurrs in the setting of cholestasis, where bile acid signaling could promote cholangiocyte growth via activation of growth factors. External stimuli such as liver fluke or viral hepatitis infections favor the induction of pro-inflammatory signals, by epithelial cells and other cells in the local microenvironment. The subsequent release of growth-promoting factors and cytokines, e.g., IL-6 and TGFβ promote cholangiocyte proliferation. These effects, along with the accumulation of genetic and epigenetic alterations in oncogenes and tumor suppressor genes lead to the malignant transformation and to deregulation of key signaling pathways such as EGFR, ERBB2, HGF/MET, VEGFR. These then contribute to the hallmarks of cancer such as proliferation, survival, invasion and enhanced angiogenesis. Alterations in pathways such as those involving the reversion-inducing-cysteine-rich protein with Kazal motifs that can modulate metalloproteases may contribute to tumor spread [43].
Molecular pathogenesis of intrahepatic cholangiocarcinoma. Promotion of tumor development follows chronic biliary tract inflammation, with the release of inflammatory cytokines inducing iNOS in cholangiocytes, favoring mutagenesis, impaired DNA repair and COX-II up-regulation, and cholestasis. Local microenvironmental factors leading to activation of Notch signaling may drive biliary differentiation. Once clonal proliferation is established, dysregulation of signaling pathways led by EGFR, RAS/MAPK, IL-6 and MET sustain enhanced proliferation. Specific genetic and epigenetic changes in either tumor cells or stromal cells contribute to malignant transformation as well as to evasion of apoptosis, neoangiogenesis, invasion or metastases once cancer has formed
Genetic and epigenetic factors in iCCA pathogenesis
Several studies have evaluated the role of genetic mutations in iCCA as well as their potential impact in prognosis and utility for diagnosis [33]. However, because of the small number of samples analyzed, and the mixed nature of the tumors studied, most of the data is not conclusive. Amongst the most frequent genetic mutations are activating mutations of KRAS particularly in hotspots located at codon 12. KRAS mutations may predict worse survival after hepatectomy although these data need to be validated in independent cohorts of samples. BRAF and EGFR mutations have also been reported but are less common. On the other hand, NRAS or PI3K mutations are rather rare in iCCA. A large number of TP53 loss-of-function mutations have been reported in iCCA and this tumor suppressor has been linked to iCCA in experimental animal models.
Human cancers can exhibit aberrant epigenetic regulation through promoter hypermethylation. Aberrant methylation of several genes including the tumor suppressor genes such as p16, RASSF1A and APC has been examined in iCCA. Promoter hypermethylation of SOCS-3, which is implicated in IL-6/STAT3 activation, has been noted in 27 % of CC tumors. Other relevant aberrantly methylated genes include RUNX3, altered in 42 % of iCCA, and p14ARF, which prevents TP53 degradation and, hence, cell cycle arrest, which has been noted to be altered in 18 % of tumors.
Recent evidence suggests that the expression of non-coding RNAs such as miRNAs may be important in iCCA. Studies evaluating the function of single oncogenic miRNAs, such as mir-214 and mir-21 have been reported. Furthermore, a unique 38-miRNA profile has been identified in a cohort of 27 iCCAs, and some of them are associated with aberrant signaling pathways such as HGF/MET, IL-6, etc. More recently, a link between miR-200c, stem cell traits and poor prognosis has been proposed. Overall though, the exact role of miRNAs either as oncoMIRs or as prognostic markers remains to be elucidated.
The iCCAs are desmoplastic cancers frequently surrounded by a dense stroma with marked cellular admixture. Studies on the role for cancer-associated fibroblasts in the growth and invasion of cholangiocarcinoma suggest that targeting molecular signals released from cancer-associated fibroblasts may be useful for the treatment of cholangiocarcinoma.
Deregulated cell signaling pathways
Several intracellular signaling pathways have been found to be deregulated in iCCA. These include signaling pathways involved in responses to growth factors such as EGF, HGF/MET, VEGF and KRAS/MAPK or Interleukin-6 (IL-6)/STAT [33]. Other emerging pathways, including Hedgehog, WNT/catenin, and Hippo have been only occasionally described in iCCA.
IL-6 is released by tumor cells in inflammatory signaling, and can contribute to the growth of malignant cholangiocytes through autocrine or paracrine mechanisms. IL-6 has been shown to have several other effects such as increased telomerase, altered methylation of growth factor receptors and altered miRNA expression that can contribute to tumor genesis or behavior [44, 45]. The over-expression of IL-6 observed in cholangiocarcinoma may result from epigenetic silencing of SOCS-3 [46]. Targeting the IL-6 dependent phenotype defined by genetic changes has been used to identify potential new therapeutics [47].
Members of the EGFR family, EGFR and ERBB2, have been implicated in iCCA pathogenesis. Although mutations in EGFR family members are infrequent, overexpression of receptors occurs in 10–32 %. An oncogenic role has been shown in a tissue-specific transgenic model that develops iCCA in ~30 %. Aberrant phosphorylation of EGFR receptors activates MAPK/ERK and p38, which can increase COX-2 and inhibits apoptosis as well as promotes tumor growth. In vitro, blocking EGFR with erlotinib can decrease cholangiocarcinoma cell proliferation. On the other hand, growth inhibition in vivo is noted with lapatinib and requires blocking both ERBB1 and ERBB2 receptors.
Another pathway that is less well established but may be important is the HGF/MET pathway. MET is a key regulator of invasive growth. Interaction of HGF and its receptor MET can activate many pathways including MAPK, PI3K and STAT. Overexpression of MET occurs in 12–58 % of cases of iCCA and has been linked to overexpression of members of the EGFR family and has shown the capacity of HGF to stimulate migration and invasion in CC cells.
VEGF and angiogenic signaling may also be important. Alterations in VEGF occur in almost 50 % of iCCA and correlate with a poor prognosis. Sorafenib has anti-tumor effects in vitro and in vivo and is a mixed kinase inhibitor that can act against BRAF and VEGFR.
Developmental pathways such as Notch signaling are implicated in cholangiocarcinoma as discussed above. Other pathways such as Wnt/-beta catenin pathways may be of importance, although genetic mutations in beta-catenin, axin 1 and APC are rare, and few studies have shown aberrant nuclear localization of beta catenin in iCCA. Thus, the Wnt-beta catenin pathway may not be as major a contributor to iCCA as it is to HCC.
Although many of these signaling pathways contain potential drivers of carcinogenesis that could be targeted for the treatment of iCCA, no oncogenic addiction loops have been documented. The experience of molecular targeted therapies in many preclinical studies has been disappointing.
Targeted therapies for biliary tract cancers
Several agents have been evaluated either singly or in combination for the treatment of iCCA and other biliary cancers [33, 48–56]. These include studies of molecular targeted therapies such as sorafenib, erlotinib, sunitinb, and selumetinib. Most of these studies are phase II studies. Single agent studies have reported a median overall survival ranging from 4.4 to 9.8 months. Combination approaches, e.g., with the use of gemcitabine and cisplatin or gemcitabine, oxaliplatin and cetuximab have resulted in an increase in median overall survival from 9.5 to 15.2 months. Caution is needed in interpreting these results for patients with iCCA because most of these studies are based on small numbers of patients that have included mixed populations of biliary cancers. There are several ongoing clinical trials using targeted therapy with conventional chemotherapy. There is very little preclinical data published in the literature to justify many of these combinations and the results of the trials are awaited. In the future, molecular profiling may be useful to select suitable rational therapies for iCCA [57].
Conclusion
In this overview, we have discussed some recent advances in the pathogenesis of iCCA. New knowledge is being generated from epidemiological studies, genome wide profiling studies, and laboratory based investigations. The emerging data provide several new insights into risk factors, genomic composition, cellular origins and contribution of the tumor microenvironment to the pathogenesis of these cancers. The insights provided by these studies provide improved understanding of the molecular pathogenesis as well as the potential for developing new approaches for the detection, diagnosis and therapy of these devastating cancers.
References
Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8(4):189–200.
Gatto M, Bragazzi MC, Semeraro R, Napoli C, Gentile R, Torrice A, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42(4):253–60.
Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10 (Epub 2002/05/07).
von Hahn T, Ciesek S, Wegener G, Plentz RR, Weismuller TJ, Wedemeyer H, et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroenterol. 2011;46(9):1092–8 (Epub 2011/06/23).
Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–7 (Epub 2001/06/08).
Braconi C, Patel T. Cholangiocarcinoma: new insights into disease pathogenesis and biology. Infect Dis Clin North Am. 2010;24(4):871–884, vii (Epub 2010/10/13).
Miller G, Socci ND, Dhall D, D’Angelica M, DeMatteo RP, Allen PJ, et al. Genome wide analysis and clinical correlation of chromosomal and transcriptional mutations in cancers of the biliary tract. J Exp Clin Cancer Res CR. 2009;28:62 (Epub 2009/05/14).
Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102(5):1016–21 (Epub 2007/02/28).
Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang Y, et al. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma—focus on East and South-Eastern Asia. Asian Pac J Cancer Prev APJCP. 2010;11(5):1159–66 (Epub 2011/01/05).
Razumilava N, Gores GJ. Classification, Diagnosis, and Management of Cholangiocarcinoma. Clin Gastroenterol Hepatol. 2012 (Epub 2012/09/18).
Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(9):512–22 (Epub 2011/08/03).
Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepato Biliary Pancreat Surg. 2003;10(4):288–91 (Epub 2003/11/05).
Sempoux C, Jibara G, Ward SC, Fan C, Qin L, Roayaie S, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis. 2011;31(1):49–60 (Epub 2011/02/24).
Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2(12):419–27 (Epub 2010/12/31).
Cardinale V, Semeraro R, Torrice A, Gatto M, Napoli C, Bragazzi MC, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol. 2(11):407–16.
Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54(1):173–84 (Epub 2011/04/14).
Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: the carcinogenic human liver fluke. WJG. 2008;14(5):666–74 (Epub 2008/01/22).
Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996;25(5):933–40 (Epub 1996/10/01).
Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117(5):854–60 (Epub 2005/06/16).
Soreide K, Korner H, Havnen J, Soreide JA. Bile duct cysts in adults. British J Surg. 2004;91(12):1538–48 (Epub 2004/11/19).
Kumagai S, Kurumatani N, Arimoto A, Ichihara G. Cholangiocarcinoma among offset colour proof-printing workers exposed to 1,2-dichloropropane and/or dichloromethane. Occup Environ Med. 2013;70(7):508–10 (Epub 2013/03/16).
Brandi G, Di Girolamo S, Farioli A, de Rosa F, Curti S, Pinna AD, et al. Asbestos: a hidden player behind the cholangiocarcinoma increase? Findings from a case-control analysis. CCC. 2013;24(5):911–8 (Epub 2013/02/15).
Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. CCC. 2001;12(10):959–64 (Epub 2002/01/26).
El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of U.S. veterans. Hepatology. 2009;49(1):116–23 (Epub 2008/12/17).
Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103(7):1716–20.
Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128(3):620–6 (Epub 2005/03/15).
Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004;95(7):592–5 (Epub 2004/07/13).
Yu TH, Yuan RH, Chen YL, Yang WC, Hsu HC, Jeng YM. Viral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and N-cadherin expression. Mod Pathol. 24(6):810–9.
Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. WJG. 2008;14(4):632–5 (Epub 2008/01/19).
Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76 (Epub 2012/03/17).
Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142(4):1021–1031 e15 (Epub 2011/12/20).
Andersen JB, Thorgeirsson SS. Genetic profiling of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2012;28(3):266–72 (Epub 2012/03/08).
Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013 (Epub 2013/01/16).
Woo HG, Lee JH, Yoon JH, Kim CY, Lee HS, Jang JJ, et al. Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res. 2010;70(8):3034–41 (Epub 2010/04/17).
Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, et al. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of mir-200c and EMT in intrahepatic cholangiocarcinoma. Hepatology. 2012 (Epub 2012/06/19).
Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12(4):410–6 (Epub 2006/03/15).
Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144(4):829–40 (Epub 2013/01/09).
Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9(1):44–54 (Epub 2011/12/07).
Wu YM, Su F, Kalyana-Sundaram S, Khazanov N, Ateeq B, Cao X, et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3(6):636–47 (Epub 2013/04/06).
Zender S, Nickeleit I, Wuestefeld T, Sorensen I, Dauch D, Bozko P, et al. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23(6):784–95 (Epub 2013/06/04).
Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122(8):2911–5 (Epub 2012/07/17).
Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012 (Epub 2012/10/02).
Namwat N, Puetkasichonpasutha J, Loilome W, Yongvanit P, Techasen A, Puapairoj A, et al. Downregulation of reversion-inducing-cysteine-rich protein with Kazal motifs (RECK) is associated with enhanced expression of matrix metalloproteinases and cholangiocarcinoma metastases. J Gastroenterol. 2011;46(5):664–75 (Epub 2010/11/16).
Meng F, Wehbe-Janek H, Henson R, Smith H, Patel T. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene. 2008;27(3):378–86 (Epub 2007/07/11).
Wehbe H, Henson R, Meng F, Mize-Berge J, Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66(21):10517–24 (Epub 2006/11/03).
Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132(1):384–96 (Epub 2007/01/24).
Braconi C, Swenson E, Kogure T, Huang N, Patel T. Targeting the IL-6 dependent phenotype can identify novel therapies for cholangiocarcinoma. PloS one. 2010;5(12):e15195 (Epub 2010/12/24).
Stuebs P, Habermann P, Garlipp B, Schuette K, Zierau K, Lippert H, et al. Developments of treatment of advanced intrahepatic cholangiocarcinoma: an analysis of systemic and local therapy modes in 57 patients. J Clin Oncol. 2012;30(Suppl 4):abstr 346.
Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7(6):593–600.
Kornek GV, Schuell B, Laengle F, Gruenberger T, Penz M, Karall K, et al. Mitomycin C in combination with capecitabine or biweekly high-dose gemcitabine in patients with advanced biliary tract cancer: a randomised phase II trial. Ann Oncol. 2004;15(3):478–83.
Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, et al. A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41(3):398–403 (Epub 2005/02/05).
Rao S, Cunningham D, Hawkins RE, Hill ME, Smith D, Daniel F, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared to epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br J Cancer. 2005;92(9):1650–4.
Takada T, Nimura Y, Katoh H, Nagakawa T, Nakayama T, Matsushiro T, et al. Prospective randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C for non-resectable pancreatic and biliary carcinoma: multicenter randomized trial. Hepatogastroenterology. 1998;45(24):2020–6 (Epub 1999/02/10).
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N Engl J Med. 2010;362(14):1273–81.
Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study—The UK ABC-01 Study. Br J Cancer. 2009;101(4):621–7 (Epub 2009/08/13).
Sugiyama H, Onuki K, Ishige K, Baba N, Ueda T, Matsuda S, et al. Potent in vitro and in vivo antitumor activity of sorafenib against human intrahepatic cholangiocarcinoma cells. J Gastroenterol. 2011;46(6):779–89 (Epub 2011/02/19).
Voss JS, Holtegaard LM, Kerr SE, Fritcher EG, Roberts LR, Gores GJ, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Human pathology. 2013 (Epub 2013/02/09).
Acknowledgments
The preparation of this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK069370. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, T. New insights into the molecular pathogenesis of intrahepatic cholangiocarcinoma. J Gastroenterol 49, 165–172 (2014). https://doi.org/10.1007/s00535-013-0894-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0894-y