Abstract
Purpose
Cancer and cancer therapy-related neurocognitive changes negatively affect quality of life, yet few studies have examined neurocognitive changes in patients with head and neck cancer. The purpose of this study was to evaluate neurocognitive performance in patients with head and neck cancer at baseline before starting treatment and 3 months after treatment completion to assess treatment-associated changes in performance.
Methods
Patients with head and neck cancer who were to receive primary or adjuvant chemoradiation (N = 55) underwent neuropsychological testing before and 3 months posttreatment. Changes in neurocognitive performance were assessed using a practice effect adjusted Reliable Change Index.
Results
At baseline, 38 % of patients exhibited global neurocognitive impairment. Posttreatment, 21.8 % demonstrated declines in neurocognitive performance in at least one domain. Declines in domain-specific performance ranged from 1.8 to 12.7 % with the greatest decline in language, specifically verb retrieval. Domain-specific improvements ranged from 0 to 7.3 %.
Conclusions
Patients had a high prevalence of baseline neurocognitive impairment. While neurocognitive performance posttreatment remained unchanged in the majority, almost 13 % suffered declines in language. Small percentages of patients exhibited improvements in their performance. Long-term effects and risk factors for neurocognitive decline in this population should be studied on a larger scale.
Similar content being viewed by others
Introduction
Patients with cancer experience neurocognitive deficits associated with cancer and cancer treatment [1–3]. The neurocognitive domains most commonly affected are attention, memory, and executive functioning. Deficits in these domains may negatively affect patients’ abilities to understand and follow complex treatment regimens. They may also adversely impact patients’ activities of daily living, engagement in social activities, and work productivity, thus diminishing their quality of life [4–7].
Data on neurocognitive functioning in patients with head and neck cancer (HNC) are primarily limited to eight small studies [8–15]. Six studies [8–13] evaluated the neurocognitive effects of potential radiation-induced brain injury. The study samples included only patients with either nasopharyngeal or paranasal sinus tumors, in which treatment entailed incidental radiation to normal brain tissue and thus the potential for direct radiation damage. These samples do not reflect most HNC patients who have oral cavity, oropharynx, hypopharynx, and larynx tumors, where brain tissue is seldom included in the radiation field. The studies also have other methodological limitations. Five studies [8, 9, 11–13] used cross-sectional designs with only posttreatment neurocognitive assessments with varied control groups and inconsistent posttreatment assessment time points ranging from 7 days to 30 years.
Today, combined modality therapy, including concurrent chemoradiation (CCR), is commonly used to treat locally advanced HNC. Most of the aforementioned studies used radiation as a single treatment modality and, therefore, fail to reflect potential neurocognitive changes associated with combined modality therapy. Treatment regimens using CCR may result in increased neurocognitive dysfunction due to the neurotoxic effects of chemotherapy and/or the enhancement of radiation-induced toxicity.
Two recent studies [14, 15] included patients with non-nasopharyngeal tumors treated with contemporary treatment regimens. In a prospective cross-sectional study, Gan et al. [14] examined neurocognitive function in 10 patients 20 months after treatment on average (range = 9–41 months). Five patients were treated with radiation as a single modality, and five patients were treated with CCR. Change in neurocognitive function was estimated based on comparison to patients’ z-scores for IQ. Nine patients demonstrated impairment across multiple domains, with memory being most affected. A trend suggested that patients receiving CCR had increased neurocognitive dysfunction. Abdul Razak et al. [15] reported that out of 24 HNC patients treated with radiation with or without cisplatin or panitumumab, 25 % had a decline in at least one neurocognitive domain and 12.5 % had a decline in multiple domains. The most affected domains were memory and attention. Twenty percent had improved neurocognitive function in at least one domain. Of note, 46 % reported a decline in their perceived neurocognitive function.
Limited data support that some HNC patients exhibit posttreatment neurocognitive deficits. The most commonly affected neurocognitive domains include visual and verbal memory, executive function, attention/concentration, language, and visuospatial ability. We conducted a prospective, longitudinal study to assess neurocognitive functioning in a cohort of HNC patients who were planned to receive primary or adjuvant CCR. Assessments were conducted before treatment and 3 months posttreatment to evaluate changes in neurocognitive performance. We previously reported on baseline neurocognitive function in the enrolled sample (N = 70) [16]. Almost half (47 %) of the patients exhibited neurocognitive impairment based on a global deficit score. The neurocognitive domain with the highest rate of baseline impairment was verbal learning (35.7 %), followed by executive function (31.8 %), verbal memory (30.3 %), processing speed (27.1 %), language (24.3 %), and attention/concentration (23.0 %). Here, we report changes in neurocognitive performance between baseline and 3 months posttreatment in those patients with complete baseline and posttreatment data (N = 55).
Methods
Sample and setting
The sample included adults (≥21 years) with histologically confirmed HNC who were to receive either primary or adjuvant CCR or induction chemotherapy followed by CCR. Other eligibility criteria included (1) ability to hear, understand, and speak English, and (2) ability and willingness to provide informed consent. Exclusion criteria included (1) prior history of cancer except for basal cell or surgically treated squamous cell skin cancer, (2) known brain metastasis, and (3) planned prophylactic intracranial radiation.
Patients were recruited from the Vanderbilt-Ingram Cancer Center (VICC) and the Nashville General Hospital at Meharry. All eligible patients who provided written informed consent were enrolled consecutively into the study. The study was approved by the Institutional Review Boards at Vanderbilt University and Meharry Medical College and by the VICC Scientific Review Committee.
Procedures
Patients were assessed with a brief neuropsychological test battery and with neurocognitive function, symptom distress, and mood measures (Table 1). Baseline testing and other measures were completed before the initiation of induction chemotherapy or CCR. Follow-up testing was scheduled 3 months posttreatment. A delay was allowed if medical issues precluded a patient’s ability to complete testing. Neuropsychological tests were administered and scored in a standardized manner by trained research staff. For participant convenience, study assessments were done during scheduled clinic visits.
Sociodemographic data, smoking history, alcohol misuse based on the Alcohol Use Disorders Identification Test (AUDIT) [26], and comorbid medical conditions were obtained at baseline by patient self-report. Disease characteristics, treatment data, and other clinical data (e.g., prior psychiatric diagnosis, current opioid prescription, and Eastern Cooperative Oncology Group [ECOG] performance status) were abstracted from patients’ medical records.
Methods for defining neurocognitive impairment
Raw scores on neuropsychological tests were converted to T-scores using published normative data adjusted for age, education, gender, and race as appropriate. T-score distributions have a normative mean of 50 and a standard deviation of 10 with higher scores reflecting better performance. Heaton et al.’s [27] performance classification system was used to classify the level of impairment for each patient in each neurocognitive domain; T-scores >40 indicate normal performance or no impairment, 35–39 mild impairment, 30–34 mild to moderate impairment, 25–29 moderate impairment, 20–24 moderate to severe impairment, and <19 severe impairment.
A global deficit score (GDS) [28] was computed by converting T-scores on individual neuropsychological tests to deficit scores and calculating an average. Using the levels of impairment described above, T-scores for each measure were assigned corresponding deficit scores ranging from 0 (no impairment) to 5 (severe impairment). Each patient’s deficit scores were summed and then divided by the total number of measures to compute a GDS. A GDS >0.5 has been used as a cut point for determining global neurocognitive impairment [28–30]. A GDS >0.5 is approximately equivalent to averaging mild impairment on 50 % of tests.
Method for defining neurocognitive change
To evaluate changes in neurocognitive performance from baseline to posttreatment, we used a Reliable Change Index (RCI) [31] adjusted for practice effects [32]. Using the equation of Chelune et al. [32], published data from normative samples were used to calculate practice effect adjusted RCI values. This involved calculating a predicted retest score as the participant’s difference score (posttreatment score–baseline score) minus the mean practice effect of the normative sample, the sum of which was then divided by the standard error of the difference for the test. The 90 % confidence level or critical t-value of 1.674 was used to indicate that the posttest score reflected more than simple fluctuations in the imprecise measurements and test–retest phenomenon. Thus, if an observed RCI value was >−1.674, then it was concluded that a reliable decrease in performance had occurred; if the RCI value was >+1.674, then improvement was indicated.
Statistical analysis
Frequency distributions were used to summarize the nominal and ordinal demographic and clinical characteristics of the patients. Means and standard deviations were used to present summaries for age, estimated IQ, and MMSE score. Median, minimum, and maximum values summarize number of co-morbidities. Due to the skewed distributions of neuropsychological test scores, median values were used as indicators of central tendency; the 25th and 75th inter-quartile range (IQR) boundaries describe variability. Comparisons between baseline characteristics and neuropsychological test scores for those patients completing the posttreatment tests and those who did not were conducted using the chi-square test of independence (nominal, ordinal data), independent t-tests (age, estimated IQ, MMSE), and Mann–Whitney tests (baseline neuropsychological test scores). Counts and percentages were used to summarize neurocognitive impairment and changes in neurocognitive performance. Tests of differences in changes in the rates of impairment from baseline to posttreatment were conducted using McNemar tests; tests of changes in neuropsychological test scores used Wilcoxon signed-ranks tests.
Spearman’s rho correlations were performed to assess the strength and direction of associations between sociodemographic, clinical, symptom distress, and mood variables and the standardized neurocognitive performance measures at each time point. The Kruskal–Wallis test was used to evaluate associations of smoking history with the neurocognitive performance measures. Because of the large number of associations examined, an alpha level of 0.01 was used to establish statistical significance. All other tests used an alpha value of 0.05 for determination of statistical significance.
Results
Figure 1 shows a schematic of participant recruitment and follow-up. Seventy of 99 (70 %) eligible patients who were approached enrolled in the study and underwent neurocognitive testing before treatment. Five patients died during treatment or after treatment completion. Two patients declined to complete the posttreatment assessment and two were lost to follow-up. An additional two patients transferred their care to another facility and did not return for follow-up. Four patients did not have complete baseline or posttreatment data and were excluded from the analysis. Thus, 55 patients (78.6 % of those enrolled) had complete baseline and posttreatment data on all neurocognitive performance measures and were included in the final analysis. The interval between the baseline and posttreatment assessments ranged from 4.4 to 10.8 months (median 6.9). Posttreatment assessments were conducted a median of 3.4 months after treatment completion (range = 2.4–5.9 months).
Patient characteristics
Summaries of the sociodemographic and clinical characteristics of patients who completed both pretreatment and posttreatment assessments (n = 55) and those who did not complete the posttreatment assessment (n = 15) are shown in Table 2. Patients who completed both assessments were mostly male (78.2 %), Caucasian (90.0 %), married or partnered (80.0 %), and had a least a high school education (89.1 %). Average age was 55.1 years (range 33–70). The majority had oropharyngeal tumors (54.5 %) and Stage IV disease (78.2 %). At baseline, patients were prescribed the following classes of medications with potential psychoactive effects: opioids (n = 23), anxiolytics (n = 10), antidepressants (n = 8), and sedative/hypnotics (n = 1). At the time of the posttreatment assessment, patients were prescribed the following: opioids (n = 34), anxiolytics (n = 10), antidepressants (n = 14), and sedative/hypnotics (n = 5).
Compared to patients who completed the posttreatment assessment, a statistically significant higher proportion of patients who did not complete the assessments had AUDIT scores indicating problematic alcohol use (scores > 8; 5/15 or 33.3 % vs 5/55 or 9.1 %; p = 0.031). Patients who did not complete the posttreatment assessment also had statistically significant lower baseline scores on the MMSE (mean 26.9 vs 28.3, p = 0.002), verbal learning (RAVLT-total) (median 39.1 vs 46.9, p = 0.002), attention/concentration (TMT-A) (median 40.5 vs 48.0, p = 0.022), executive function (TMT-B) (median 36.0 vs 47.0, p = 0.041), and processing speed (SDMT) (median 37.9 vs 48.1, p = 0.002).
Neurocognitive performance at baseline
Summaries of neuropsychological test scores and rates of impaired performance in specific neurocognitive domains at baseline and posttreatment are presented in Table 3. At baseline, deficits were noted in all domains; the prevalence of deficits ranged from 18.2 to 30.9 %. The highest rate of impairment was noted in verbal learning. The prevalence of moderate to severe impairment ranged from 1.8 to 9.1 % with the highest rate of moderate to severe impairment noted in verbal memory. Sixteen patients (29.1 %) showed at least mild impairment in one domain, 12 (21.8 %) in two domains, 4 (7.3 %) in three domains, 4 (7.3 %) in four domains, and 2 (3.6 %) in five domains. Based on the GDS, 38.2 % of patients demonstrated global neurocognitive impairment at baseline.
Very few sociodemographic, clinical, symptom distress, or mood variables were correlated with standardized neurocognitive performance scores at baseline. Smoking history was statistically significantly associated with processing speed (X 2 (df = 2) = 15.01, p = 0.001) and the GDS (X 2 (df = 2) = 9.44, p = 0.009). An inverse association was noted between processing speed and physical symptom distress (r s = −0.38, p = 0.004) as well as with scores on the POMS-SF Confusion Subscale (r s = −0.38, p = 0.004). There were no statistically significant associations between any of the other variables and standardized neurocognitive performance scores at baseline.
Posttreatment neurocognitive performance
Posttreatment deficits were noted in all domains; the prevalence of deficits ranged from 7.3 to 30.9 % (Table 3). The highest rate of impairment was in language. The prevalence of moderate to severe impairment ranged from 0 to 5.5 %. At the posttreatment assessment, 11 patients (20.0 %) demonstrated at least mild impairment in one domain, 9 (16.4 %) in two domains, 4 (7.3 %) in three domains, 3 (5.5 %) in four domains, and 2 (3.6 %) in five domains. Posttreatment, 25.5 % exhibited global neurocognitive impairment according to the GDS (McNemar binomial, p = 0.118).
Smoking history was statistically significantly associated with posttreatment neurocognitive performance in processing speed (X 2 (df = 2) = 10.00, p = 0.007), verbal learning (X 2 (df = 2) = 10.16, p = 0.006), and the GDS (X 2 (df = 2) = 10.47, p = 0.005). Perceived cognitive function was significantly associated with executive function (r s = −0.40, p = 0.002) and the GDS (r s = 0.40, p = 0.003). There were no statistically significant associations between posttreatment neurocognitive performance scores and any of the other sociodemographic, clinical, symptom distress, or mood variables (e.g., fatigue, depression, and anxiety).
Changes in neurocognitive performance from baseline to 3 months posttreatment
The majority (54.5 %) of patients did not demonstrate any significant change between their baseline and posttreatment performance in any of the six neurocognitive domains. Twelve patients (21.8 %) had a decline in at least one domain. Fourteen (25.4 %) patients exhibited an improvement in at least one domain. One patient demonstrated a decline in one domain and improvement in another. Comparing median scores from baseline to posttreatment (Table 3), statistically significant improvements were noted in the following domains: attention/concentration (z = 2.16, p = 0.031), executive function (z = 2.46, p = 0.014), and verbal learning (z = 3.29, p = 0.001).
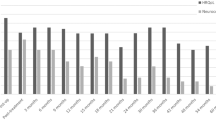
Figure 2 shows the frequency of changes in performance based on the practice effect adjusted RCI for each neurocognitive domain. Declines in domain-specific performance were noted in a small percentage of patients ranging from 1.8 to 12.7 %, with the greatest frequency of decline in language. Ten patients declined in one domain. One patient declined in two domains and another declined in three domains. Similarly, a small percentage of patients demonstrated improvement in their domain-specific performance from baseline. Domains showing the greatest frequency of improvement included attention/concentration (7.3 %), language (7.3 %), and verbal memory (7.3 %). Thirteen patients improved in one domain and one patient showed improvement in three domains.
Five patients who exhibited declines in neurocognitive performance from baseline to posttreatment had posttreatment scores that remained within the normal range (T-score > 40). An example would be a patient who had a baseline AVF score of 73 and a posttreatment score of 56. This patient demonstrated a decline in performance on the action (verb) fluency test from baseline to posttreatment, but the posttreatment score did not indicate impairment in this neurocognitive domain. One of the five patients had posttreatment declines in three domains; however, the posttreatment test scores for two domains, verbal learning and processing speed, remained in the normal range.
Discussion
In this study, we assessed neurocognitive performance in HNC patients before treatment and at 3 months posttreatment to evaluate treatment-associated changes. As we previously reported [16], a high prevalence of neurocognitive impairment was noted in patients at baseline. The majority of patients demonstrated little change in their neurocognitive performance between baseline and 3 months posttreatment. Small percentages of patients showed improvements or declines in their performance. Larger studies with longer term follow-up and comparison groups are needed to more fully investigate patterns of change in neurocognitive performance in HNC patients and to identify factors associated with neurocognitive decline.
Nearly 13 % of patients exhibited declines in language, specifically action (verb) fluency or verb retrieval. Small numbers of patients had declines in processing speed, verbal memory, verbal learning, executive function, and attention/concentration. Declines in these domains, particularly verb retrieval and generation, are indicative of frontal–subcortical pathologies [33–35]. Studies of neurocognitive function in other cancer populations also indicate a frontal–subcortical toxicity profile, with neurocognitive dysfunction within domains of information processing speed, attention, memory retrieval, and executive function [36]. Impairments in these domains may affect patients’ abilities to learn new information, reason, plan, organize, focus, and concentrate.
With the greatest decline occurring in language, the effects of HNC treatment on speech must be considered as a potential confounding factor. None of the participants in our study exhibited noticeable speech impairment or dysarthria requiring speech therapy consultation. However, the effects of other treatment-related issues such as pain and xerostomia on speech production and the ability to speak fluently cannot be ruled out. Consistent with our findings, Hsiao et al. [10] noted significant declines in language abilities and list-generating fluency in a sample of patients with nasopharyngeal cancer after intensity-modulated radiation therapy.
Tobacco smoking is a well-established risk factor for both HNC [37] and impaired neurocognitive function [38–40]. In the current study, smoking history was associated with global neurocognitive performance and domain-specific performance at baseline and posttreatment. At baseline, compared to those who never smoked, current smokers exhibited more global neurocognitive impairment and greater impairment in processing speed. Similar posttreatment associations were noted. Compared to those who never smoked, current smokers demonstrated more global neurocognitive impairment and greater impairments in processing speed and in verbal learning. HNC patients with a smoking history may be at increased risk for impaired neurocognitive function before and after treatment.
Unlike other published studies, this study evaluated changes in neurocognitive performance during the early posttreatment period. Studies of neurocognitive function in HNC patients [8–15, 41, 42] have demonstrated that problematic neurocognitive changes are common during treatment and in the late posttreatment period. In most posttreatment studies, assessments were conducted at least 1 year after treatment. Findings from these studies have shown evidence of impaired performance in multiple neurocognitive domains. Thus, HNC patients may be at increased risk for acute changes in neurocognitive function during treatment and for detrimental neurocognitive effects months after treatment. Late-onset neurocognitive decline has been reported in other cancer populations. For example, in a sample of breast cancer patients, Wefel et al. [43] found that 61 % of patients exhibited long-term neurocognitive decline posttreatment. While 71 % of these patients had persistent impairment following treatment, 29 % exhibited a delayed onset decline.
Reporting on individuals with below normal performance does not take into account patients with a significant decline within the normal range of neurocognitive performance. Individuals scoring in the average or low normal range posttreatment who previously scored in the high normal range may perceive a considerable loss in abilities. Clinically, we have observed that patients with high levels of neurocognitive performance pretreatment are distressed by perceived neurocognitive impairment posttreatment; however, upon testing, no deficits are identified. Our results suggest that a small cohort of patients demonstrated a decline in neurocognitive performance posttreatment but remained within the normal range for performance. These previously high functioning individuals may experience profound distress associated with declines in their neurocognitive performance. At the posttreatment assessment, perceived cognitive function was positively associated the GDS, indicating a relationship between perceived cognitive function and neurocognitive performance. Neurocognitive performance is typically evaluated after treatment in patients with neurocognitive complaints. This subset of patients would not demonstrate neurocognitive impairment based on their posttreatment scores.
Importantly, baseline neurocognitive impairment in HNC patients may be associated with increased risk for poor treatment outcomes. While we did not correlate neurocognitive impairment with oncological or other clinical outcomes, we observed that patients who failed to complete the posttreatment assessment had lower levels of neurocognitive performance at baseline. The reason for this is unclear. Two potential explanations may be proposed: (1) Poor neurocognitive function may correlate with poor overall health and higher rates of co-morbidities which place patients at increased risk for complications of therapy, including death, or (2) decreased neurocognitive function may impair the patient’s ability to understand and comply with complicated multi-modality therapy. It is not feasible or clinically appropriate to conduct extensive neurocognitive testing on all HNC patients before initiating treatment; however, clinicians should be aware that some patients may have baseline impairment. These patients may require more intensive follow-up and support during treatment. Clinicians should evaluate patients’ understanding of their treatments, supportive care requirements, and the adequacy of caregiver support. Future studies of neurocognitive functioning in patients with HNC should examine the effects of baseline neurocognitive function on treatment and other clinical outcomes such as radiation and chemotherapy dose intensity, symptom burden, health services utilization, and caregiving needs.
Limitations
To our knowledge, this is the largest reported prospective study examining changes in neurocognitive performance in HNC patients; however, it has a number of limitations that must be considered. First, the sample is heterogeneous. The patients underwent treatment regimens with different chemotherapeutic agents and schedules. The sample included patients with nasopharyngeal and paranasal sinus tumors who are at risk for receiving incidental brain irradiation during treatment. Patients with psychiatric illnesses were not excluded. At the time of the assessments, some patients were prescribed opioids and other medications with potential psychoactive effects. Following treatment, some patients had persistent disease. We examined associations between multiple sociodemographic, clinical, symptom distress, and mood variables and neurocognitive performance, and few significant associations were noted. Although reflective of the changing demographic of HNC patients, the sample is relatively young and may not be representative of the classic HNC population which is older [44]. Neurocognitive function in older and younger HNC patients may differ. We did not examine differences in neurocognitive performance in patients with HPV-positive and HPV-negative tumors. In future studies, it would be important to evaluate whether neurocognitive functioning differs in these populations. We used a brief neuropsychological test battery to evaluate neurocognitive performance in six domains. The neuropsychological tests were chosen for their sensitivity, ease of administration, brevity, and appropriateness for use in an ill population. To minimize participant burden, we only used one test for each neurocognitive domain. We also did not use alternate test forms with repeated administration. There was no control or comparison group. Published normative data from healthy controls were used to determine practice effects in calculating the RCI. There may be concerns about the lack of similarities between samples, differences in the timing between measurements, and variability in test administration. There was also variable timing of posttreatment assessments ranging from 2.4 to 5.9 months after treatment completion. Because of this wide timeframe for posttreatment assessments, neurocognitive performance could have been affected by variability in functional recovery after treatment. Finally, compared to those who completed the posttreatment assessment, patients who failed to complete the posttreatment assessment had poorer neurocognitive performance at baseline. Therefore, the results reported here are biased by attrition.
Conclusion
We examined changes in neurocognitive performance in HNC patients from before treatment to 3 months posttreatment. A large percentage of patients exhibited impaired neurocognitive performance before starting treatment. The majority of patients did not exhibit changes in neurocognitive performance. Small percentages of patients demonstrated either improvements or declines in specific neurocognitive domains. Declines occurred most frequently in language, processing speed, verbal learning, and verbal memory. Larger prospective longitudinal studies that include baseline assessments, serial acute and long-term posttreatment assessments, and control groups for comparison are needed to determine the extent of treatment-associated neurocognitive impairment in HNC survivors, identify modifiable risk factors, and develop interventions to mitigate neurocognitive disabilities in this understudied and vulnerable population.
References
Ahles TA, Root JC, Ryan EL (2012) Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 30:3675–3686. doi:10.1200/JCO.2012.43.0116
Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE (2003) Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literaure. J Int Neuropsych Soc 9:967–982. doi:10.1017/S1355617703970019
Vardy J, Rourke S, Tannock IF (2007) Evaluation of cognitive function associated with chemotherapy: a review of published studies and reocmmendations for future research. J Clin Oncol 25:2455–2463. doi:10.1200/JCO.2006.08.1604
Bjordal K, Kaasa S, Mastekaasa A (1994) Quality of life in patients treated for head and neck cancer: a follow-up study 7 to 11 years after radiotherapy. Int J Radiat Oncol Biol Phys 28:847–856. doi:10.1016/0360-3016(94)90104-X
Boykoff N, Moieni M, Subramanian S (2009) Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. J Cancer Surviv 3:223–232. doi:10.1007/s11764-009-0098-x
Downie FP, Mar Fan HG, Houédé-Tchen N, Yi Q, Tannock IF (2006) Cognitive function, fatigue, and menopausal symptoms in breast cancer patients receiving adjuvant chemotherapy: evaluation with patient interview after formal assessment. Psychooncology 15:921–930. doi:10.1002/pon.1035
Shilling V, Jenkins V (2007) Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs 11:6–15. doi:10.1016/j.ejon.2006.02.005
Cheung MC, Chan AS, Law SC, Chan JH, Tse VK (2000) Cognitive function of patients with nasopharyngeal carcinoma with and without temporal lobe radionecrosis. Arch Neurol 57:1347–1352. doi:10.1001/archneur.57.9.1347
Hua MS, Chen ST, Tang LM, Leung WM (1998) Neuropsychological function in patients with nasopharyngeal carcinoma after radiotherapy. J Clin Exp Neuropsychol 20:684–693. doi:10.1076/jcen.20.5.684.1131
Hsiao KY, Yeh SA, Chang CC, Tsai PC, Wu JM, Gau JS (2010) Cognitive function before and after intensity-modulated radiation therapy in patients with nasopharyngeal carcinoma: a prospective study. Int J Radiat Oncol Biol Phys 77:722–726. doi:10.1016/j.ijrobp.2009.06.080
Lam LCW, Leung SF, Chan YL (2003) Progress of memory function after radiation therapy in patients with nasopharyngeal carcinoma. J Neuropsychiatry Clin Neurosci 15:90–97
Lee PW, Hung BK, Woo EK, Tai PT, Choi DT (1989) Effects of radiation therapy on neuropsychological functioning in patients with nasopharyngeal carcinoma. J Neurol Neurosurg Psychiatry 52:488–492. doi:10.1136/jnnp.52.4.488
Meyers CA, Geara F, Wong P-F, Morrison WH (2000) Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys 46:51–55. doi:10.1016/S0360-3016(99)00376-4
Gan HK, Bernstein LJ, Brown J et al (2011) Cognitive functioning after radiotherapy or chemoradiotherapy for head-and-neck cancer. Int J Radiat Oncol Biol Phys 81:126–134. doi:10.1016/j.ijrobp.2010.05.004
Abdul Razak AR, Siu LL, Chan A et al (2011) Neurocognitive function (NCF) in patinets (pts) treated with chemo/bio-radiotherapy (C/B-RT) for head and neck cancers (HNC). J Clin Oncol 29(15 suppl):5522
Bond SM, Dietrich MS, Murphy BA (2012) Neurocognitive function in head and neck cancer patients prior to treatment. Support Care Cancer 20:149–157. doi:10.1007/s00520-010-1081-9
Reitan RM, Wolfson D (1995) Category test and trail making test as measures of frontal lobe functions. Clin Neuropsychol 9:50–56
Lezak M, Loring D (2004) Neuropsychological assessment. Oxford University Press, New York
Woods SP, Scott JC, Sires DA, Grant I, Heaton RK (2005) Action (verb) fluency: test; retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc 11:408–415. doi:10.1017/S1355617705050460
Smith A (2002) Symbol digit modalities test manual. Western Psychological Services, Los Angeles
Folstein M, Folstein S, Fanjiang G (2001) Mini-mental state examination: clinical guide and user’s guide. Psychological Assessment Resources, Lutz
Blair JR, Spreen O (1989) Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol 3:129–136. doi:10.1080/13854048908403285
Bergner M, Bobbitt RA, Carter WB, Gilson BS (1981) The sickness impact profile: development and final revision of a health status measure. Med Care 19:787–805
Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT (2000) The Memorial Symptom Assessment Scale Short Form (MSAS-SF). Cancer 89:1162–1171. doi:10.1002/1097-0142(20000901)89:5<1162::AID-CNCR26>3.0.CO;2-Y
Shacham S (1983) A shortened version of the profile of mood states. J Pers Assess 47:305–306. doi:10.1207/s15327752jpa4703_14
Babor T, Higgins-Biddle J, Suanders J, Monteiro M (2001) The Alcohol Use Disorders Identification Test. World Health Organization, Geneva
Heaton R, Miller S, Taylor M, Grant I (2004) Revised comprehensive norms for an expanded Halstead–Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources, Lutz
Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK (2004) Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26:307–319. doi:10.1080/13803390490510031
Heaton RK, Grant I, Butters N et al (1995) The HNRC 500 – Neuropsychology of HIV infection at different disease stages. J Int Neuropsychol Soc 1:231–251. doi:10.1017/S1355617700000230
Gonzales R, Heaton RK, Moore DJ et al (2003) Computerized reaction time battery versus a traditional neuropsychological batter: detecting HIV-related impairments. J Int Neuropsychol Soc 9:64–71. doi:10.1017/S1355617703910071
Jacobson NS, Truax P (1991) Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 59:12–19. doi:10.1037/0022-006X.59.1.12
Chelune GJ, Naugle RI, Luders H, Sedlak J, Awad IA (1993) Individual change after epilepsy surgery: practice effects and base-rate information. Neuropsychol 7:41–52. doi:10.1037/0894-4105.7.1.41
Piatt AL, Fields JA, Paolo A, Troster AI (1999) Action (verb naming) fluency as a unique executive function measure: convergent and divergent evidence of validity. Neuropsychol 37:1499–1503. doi:10.1016/S0028-3932(99)00066-4
Piatt AL, Fields JA, Paolo A, Koller WC, Troster AI (1999) Lexical, semantic, and action fluency in Parkinson’s disease with and without dementia. J Clin Exp Neuropsychol 21:435–443. doi:10.1076/jcen.21.4.435.885
Tekin S, Cummings JL (2002) Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 53:647–654. doi:10.1016/S0022-3999(02)00428-2
Vardy J, Wefel JS, Ahles T et al (2008) Cancer and cancer therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol 19:623–629. doi:10.1093/annonc/mdm500
Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P (2008) Tobacco smoking and cancer: a meta-analysis. Int J Cancer 122:155–164. doi:10.1002/ijc.23033
Anstey KJ, von Sanden C, Salim A, O’Kearney R (2007) Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 166:367–378. doi:10.1093/aje/kwm116
Durazzo TC, Meyerhoff DJ (2007) Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci 12:4079–4100
Chamberlain SR, Odlaug BL, Schreiber LRN, Grant JE (2012) Association between tobacco smoking and cognitive functioning in young adults. Am J Addict 21(suppl 1):S14–S19. doi:10.1111/j.1521-0391.2012.00290.x
Bond SM, Dietrich MS, Shuster JL, Murphy BA (2012) Delirium in patients with head and neck cancer in the outpatient treatment setting. Support Care Cancer 20:1023–1030. doi:10.1007/s00520-011-1174-0
Bond SM, Hawkins DK, Murphy BA (2014) Caregiver-reported neuropsychiatric symptoms in patients undergoing treatment for head and neck cancer: a pilot study. Cancer Nurs 37:227–235. doi:10.1097/NCC.0b013e31829194a3
Wefel JS, Saleeba AK, Buzdar AU, Meyers CA (2010) Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 116:3348–3356. doi:10.1002/cncr.25098
VanderWalde NA, Fleming M, Weiss J, Chera BS (2013) Treatment of older patients with head and neck cancer: a review. Oncologist 18:568–578. doi:10.1634/theoncologist.2012-0427
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Funding
This study was funded in part by the John A. Hartford Foundation Building Academic Geriatric Nursing Capacity Program through the American Academy of Nursing (AAN) and the Vanderbilt University School of Nursing Postdoctoral Program.
Additional information
Prior Presentation: Partial findings were presented at the 7th Annual Oncology Congress in San Francisco, CA, in October 2011.
Rights and permissions
About this article
Cite this article
Bond, S.M., Dietrich, M.S., Gilbert, J. et al. Neurocognitive function in patients with head and neck cancer undergoing primary or adjuvant chemoradiation treatment. Support Care Cancer 24, 4433–4442 (2016). https://doi.org/10.1007/s00520-016-3284-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-016-3284-1