Abstract
Purpose
This study assessed cardiorespiratory fitness (CRF), physical activity (PA), and sedentary behavior (SB), as well as factors associated with these outcomes in children during or shortly after cancer treatment.
Methods
Cross-sectionally, CRF data, obtained by the cardiopulmonary exercise test, and PA and SB data, obtained by an accelerometer, were assessed in children with cancer (8–18 years old). Linear regression models were used to determine associations between CRF, PA, or SB and patient characteristics.
Results
Among 60 children with cancer, mean age 12.6 years, 35 boys, 28 % were during cancer treatment. CRF, reported as the z score of VO2peak, showed that 32 children had a VO2peak z score which was −2 below the predicted value. CRF was significantly associated with PA and SB: each additional activity count per minute resulted in 0.05 ml/kg/min VO2peak increase and each additional minute sedentary reduced VO2peak by 0.06 ml/kg/min. Multiple linear regression models of PA and SB showed that decreased activity was significantly associated with higher age, being fatigued, being during childhood cancer treatment (p < 0.001), or having a higher percentage of fat mass. The multiple linear regression model showed that lower CRF was significantly associated with increased fatigue, being during cancer treatment, having a higher percentage of fat mass, and lower belief of own athletic competence (p < 0.001).
Conclusion
This study revealed that children during or shortly after cancer treatment have low CRF scores. The most inactive children had a higher fat mass, were fatigued, older, and during childhood cancer treatment. Unexpectedly, treatment-related factors showed no significant association with activity behavior.
Similar content being viewed by others
Introduction
Cardiorespiratory fitness (CRF) and muscle strength have shown to be reduced both during and after childhood cancer treatment [1–3]. Both are considered important health markers, since they represent the functional status of most body functions involved in the performance of daily physical activities (PA). A reduction in CRF and muscle strength can be caused by physical inactivity [4, 5]. When inactivity persists, it will put the patient at risk for obesity, cardiovascular disease, reduced muscle strength, decreased bone mineral density, and subsequently, a reduced health-related quality of life (HrQoL) [5–9].
In childhood cancer patients, the cancer treatment may adversely interfere with the patients’ physical and mental ability to engage in PA. Several determinants are known to influence motor function. Chemotherapy can result in anemia, decreased oxygen transport to the muscles, and reduced muscle function [10]; the use of vincristine can result in peripheral neuropathy with muscle weakness in hands and feet, while anthracyclines may impair cardiac function, and bleomycin may result in decreased lung function due to pulmonary fibrosis [11]. Also, mechanical factors are important, such as decreased motor function after an amputation in bone tumor patients [12] or ataxia following brain tumor treatment [13]. Apart from clear physical factors, being fatigued, as well as having depressive symptoms, may also negatively influence PA [14].
Previous studies, using an accelerometer to objectively measure PA, showed that childhood cancer patients have low PA levels [4, 5, 15, 16]. These studies, however, were performed in small groups and did not study the association between PA and CRF.
Only recently, both in children and adults, sedentary behavior (SB) has been introduced as a new important negative factor for health [17]. SB is defined as activities that typically require low-energy expenditure, such as sitting on the couch [18]. Frequent and prolonged sitting periods puts a person at risk for obesity and other metabolic conditions that enhance the risk of chronic diseases (e.g., type 2 diabetes, cardiovascular disease, breast and colon cancer) [19–21]. Children with cancer are already at increased risk for chronic diseases; therefore, assessing SB in children with cancer is important [22]. Through questionnaires, one study found that 9 % of the children with cancer left their bed for less than 1 h while 44 % of the questioned patients reported to leave their bed over 10 h per day during homestays [23]. Up till now, no studies objectively measured SB in childhood cancer patients.
This study aimed to assess CRF in childhood cancer patients during or shortly after treatment and to evaluate the association with objectively measured PA and SB. In addition, the impact of several physical and psychosocial factors on PA and SB was assessed in order to identify targets for future interventions aimed at stimulating PA and decreasing SB to ultimately increase CRF and HrQoL.
Methods
Study population
This study is a cross-sectional study using the baseline data of a randomized controlled trial (RCT), evaluating the effects of a combined 12-week exercise and psychosocial training program for children with cancer on physical fitness and HrQoL (the Quality of Life in Motion [QLIM] study). Details on the design of this study had been described previously [24].
Eligible children were 8–18 years old, diagnosed with any type of malignancy, treated with chemotherapy and/or radiotherapy, during or within the first year after cancer treatment. Patients who were not able to make self-reflections (children <8 years old or with a mental retardation), who received growth hormones, who were planned for stem cell transplantation, and those who were not able to ride a bike or read and write Dutch were excluded.
Patients were recruited between March 2009 and July 2013. Eligible patients were identified through patient databases by pediatric oncologists, a study-researcher, or a research nurse of the pediatric oncology/hematology departments within four university hospitals in the Netherlands: VU University Medical Center Amsterdam, Academic Medical Center Amsterdam, Erasmus MC Rotterdam, and University Medical Center, Utrecht. Patient records and the clinic data were weekly reviewed to verify eligibility. When children needed to be hospitalized, and when clinical conditions (low blood counts, infections, or others) made participation impossible (as assessed by their treating physician), children were considered unable to start study participation, and therefore the start of the study was postponed. Both patients and their parents or legal representatives received spoken and written information and provided written informed consent as by approval of the medical ethics committees of the four participating hospitals and was performed according to the 1964 Declaration of Helsinki. Register; Dutch Trial Registry number NTR1531.
Procedure
Study data were obtained at the university hospital of the child. Children were assessed on CRF, muscle strength, and body composition. Child report questionnaires were used to measure psychosocial functioning. In the week after the study measurements, an accelerometer was used to measure PA and SB. Clinical data, such as data on cancer diagnosis, treatments, and complications, were obtained from medical records.
Measures
CRF was assessed during a cardiopulmonary exercise test on an electronically braked cycle ergometer (Lode, Corival P, ProCare B.V. Groningen, the Netherlands) using the Godfrey protocol. During the test, ventilatory gas exchange data were determined breath by breath. The peak oxygen uptake (VO2peak) was calculated as the mean value of the final 30 s of the test and expressed in milliliters per kilogram per minute (ml/kg/min). Predicted values for VO2peak were calculated from an age- and sex-based equation [25]. Measured VO2peak results were compared with these predicted values.
Physical activity and sedentary behavior
PA and SB of each patient were measured by the Actical activity monitor (B series, Philips Respironics Actical MiniMitter, Murrysville, PA, USA). The Actical is an accelerometer (37 × 29 × 11 mm) which has been validated in children between 7–18 years of age [26]. The receiver operating characteristic curves were 0.85, 0.93, and 0.95 for a sedentary to light, light to moderate, and a moderate to vigorous activity level, respectively [26]. The Actical accelerometer was attached to an elastic waist belt and worn on the left hip during daytime at waking hours (between 6:00 a.m. and 11:59 p.m.) for four consecutive days (Wednesday–Saturday). The device was removed while bathing or swimming.
When the device was worn less than 500 min/day, the measurement of that day was considered invalid. Time not wearing the accelerometer was defined as 60 min of consecutive zeros on the readout, and this time during waking hours was excluded from the analyses. The acceleration signal of the Actical is summed over a specific time interval (epoch) [27]. A 15-s epoch was used in the study.
PA was expressed as mean counts per minute (cpm). For the present study, we used the following cpm range to define the different activity intensities: sedentary status corresponds with an activity count of less than 100 cpm, light activity with 100–1599 cpm, and moderate activity with 1600–4760 cpm, and 4760 or more cpm was considered as a vigorous activity level [28]. Children who participated at least 60 min per day at an activity level of >1600 cpm were categorized as fulfilling the international PA recommendations [29].
SB, defined as a cpm below 100 was presented as mean minutes sedentary (out of 1080 measured min/day) and as accelerometer-based sedentary bouts. Sedentary bouts were defined as periods of at least 5, 10, 20, 30, and 60 min of SB [17, 30].
Possible associated factors
Physical factors
For all study participants, height and weight were measured to the nearest millimeter (mm) and 0.1 kilogram (kg), respectively. Body mass index (BMI; kg/m2) was calculated as well as the BMI z scores using the growth calculator for professionals [31].
Muscle strength was measured by the use of hand-held dynamometry (CITEC, CT3001, Haren, the Netherlands) [32] using the break method [33]. The highest out of three scores were used in the analyses. Lower body muscle strength was calculated as the sum of the best (left or right) upper leg, lower leg, and foot scores. Upper body muscle strength was calculated as the sum of the best shoulder, elbow, and grip strength scores.
Body fat was assessed by dual energy X-ray absorptiometry (DXA). The assessment was performed on a Hologic Delphi/Discovery or a Lunar Prodigy scanner. Differences in percentage of fat mass were corrected in accordance with the equation of Shepherd et al. (2012) [34].
Fatigue was self-assessed by the use of the PedsQL™ Multidimensional Fatigue Scale (acute version) [35], with lower scores indicating more fatigue (range: 0–100); the results of the subscale “general fatigue” were included in the study analyses.
Psychosocial factors
The participation in sports of the study participant before the cancer diagnosis was evaluated by the use of a questionnaire which was developed for this study.
Athletic competence was assessed with a subscale of the Self Perception Profile Questionnaire for children aged 8–11 years (CBSK) and for adolescents aged 12–18 years (CBSA) [36]. Higher scores reflect a more positive perception of the athletic competence (range between 0 to 100 points).
Depressive symptoms were assessed by the use of the Children’s Depression Inventory (CDI). This questionnaire for children aged 7–18 years contains 27 items which assesses self-reported depressive symptoms [37]. For this study, we used the total scores (range 0–54).
Statistical analysis
Normality of the data was assessed by normality plots and the Shapiro-Wilk test. When data showed a normal distribution, continuous outcomes were expressed as mean (standard deviation [SD] or range), and in case of non-normal distribution, median (interquartile range [IQR]) scores were reported. Paired sample t test was used to assess differences between the observed and predicted VO2peak (ml/kg/min) values [25].
Univariate regression analyses were performed to identify association between and additional associated factors for CRF (VO2peak), PA (cpm), and SB (min sedentary/day). Because the sample size (N = 60) did not allow us to simultaneously include all potential variables into the multiple linear regression model, we preselected a maximum of six variables with p < 0.15 from the univariate regression analyses and include them in the multiple linear backward regression analyses. By hand, factors with the highest p value were removed until all factors were statistically significant. The coefficient of determination and the standard error of the estimate (SEE) are included to present a measure for variance and accuracy of the regression models. A 2-sided p value <0.05 was considered statistically significant in all analyses. IBM SPSS Statistics for Windows (Version 20.0. Armonk, NY: IBM Corp., USA) was used for the statistical analyses.
Results
General and medical characteristics
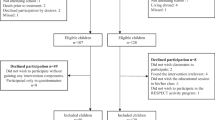
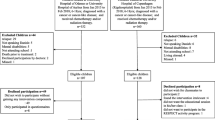
A total of 174 children were invited to participate in the QLIM study, of whom 68 (37 boys) were included (Fig. 1). Due to missing accelerometer data in eight patients, the current study therefore analyzed the results of 60 children (35 boys) with a mean age of 12.6 years (SD 3.1; range 8.0–18.0 years). A total of 17 children (28 %) were during cancer-treatment at the time of the study (Table 1). Thirty-seven (62 %) were treated with chemotherapy alone.
Both the general and medical characteristics of the eight children who were excluded from the analyses, as well as characteristics of the 106 non-participants [38], were not significantly different from the 60 children who were analyzed (data not shown).
Cardiorespiratory fitness
CRF, expressed as VO2peak (ml/kg/min) in the study population was 31.7 ml/kg/min (SD 9.2). The mean predicted value of the study group was 45.1 ml/kg/min (SD 3.6), resulting in a mean absolute difference between the measured and predicted values of −13.4 ml/kg/min (SD 9.2) (p < 0.001). Results for boys and girls separately are presented in Fig. 2a, b.
a Measured versus predicted VO2peak in boys (according to age- and sex-matched norm values) in the Quality of Life in Motion study (N = 35). b Measured versus predicted VO2peak in girls (according to age- and sex-matched norm values) in the Quality of Life in Motion study (N = 25). VO 2peak peak oxygen uptake, z score standard deviation from the mean. *Based on age-matched norm values
A total of 32 children (53 %) had a z score ≤−2, approximately 12 ml/kg/min below the predicted value [25]. The 17 children who were during treatment all belonged to the −2 z score group.
Physical activity and sedentary behavior
PA was monitored over a median period of 4 days (IQR 3.5–4 days). Overall, the median PA level was 127 cpm (IQR 80–219) (Table 2). The children spent 16 % of their daytime on light activities, were 7 % (SD 4.4) moderately active, and spent only 0.1 % (SD 0.2) of the day on a vigorous activity level.
Evaluation of SB showed that children were sedentary in 80 % of all waking hours: median of 869 min (IQR 785–911) of the 1080 min which were analyzed per day. Study results also showed that prolonged sitting periods without interruptions (≥20 or 30 min) were common (Table 2).
Cardiorespiratory fitness, physical activity/sedentary behavior, and associated factors
In either way, CRF, PA, and SB showed highly significant associations (Table 3). A positive association was found between CRF (VO2peak) and PA (cpm) (β 0.05; 95 % CI: 0.0; 0.1; p < 0.001): every additional cpm resulted in a 0.05 ml/kg/min increase in CRF. SB had a negative association with CRF (β −0.06; 95 % CI: −0.1; (−0.0); p < 0.001): every additional minute of sedentary time per day decreased the CRF by 0.06 ml/kg/min (Table 3).
Physical activity
Single factor associations showed that PA was significantly associated with the following: age (β −15.4; 95 % CI: −23.6; (−7.2); p < 0.001), percentage of fat mass (β −5.4; 95 % CI: −9.0; (−1.7); p = 0.005), being during (0)/after (1) cancer treatment (β 63.9; 95 % CI: 3.4;124.3; p = 0.039), fatigue (β 2.6; 95 % CI: 1.5; 3.7; p < 0.001), and depressive symptoms (β −6.2; 95 % CI: −11.9; (−0.5); p = 0.034) (Table 3). For the multivariate analysis, a sixth factor was added: lean body mass (β 0.0; 95 % CI: −0.0; 0.0; p = 0.056).
The multiple linear regression analysis for PA showed that age (β −11.9; 95% CI: −19.8; (−4.0); p = 0.004), being during (0)/after (1) cancer treatment (β 68.0; 95 % CI:18.1;118.0; p = 0.008), and fatigue (β 1.8; 95% CI: 0.7; 2.9; p = 0.002; i.e., higher scores indicate less fatigue) were significantly associated with PA (Table 3). Thus, younger children who were following cancer treatment and who were less fatigued were more active. These factors explained 41.8 % of the variance in PA; SEE = 85.0 (model p < 0.001).
Sedentary behavior
Significant univariate associated factors for SB (min sedentary/day) were age (β 14.6; 95 % CI: 7.8; 21.5; p < 0.001), percentage of fat mass (β 4.9; 95 % CI: 1.8; 7.9; p = 0.002), lean body mass (β 0.0; 95% CI: 0.0; 0.0; p = 0.016), fatigue (β −2.1; 95% CI:−3.1;(−1.1); p < 0.001), and depressive symptoms (β 5.8; 95 % CI: 0.9; 10.7; p = 0.021) (see Table 3). For the multiple regression analyses, also being during/after treatment (β −47.9; 95 % CI: −100.4; 4.7; p = 0.074) was added as an independent variable.
After backward elimination, the final multiple regression model for SB included age (β 10.0; 95 % CI: 3.5; 16.5; p = 0.003), percentage of fat mass (β 3.7; 95 % CI: 1.1; 6.3; p = 0.007), and fatigue (β −1.2; 95 % CI: −2.2; (−0.2); p = 0.015) (Table 3). Older age, being fatigued, and having an increased percentage of fat mass was associated with more minutes of SB per day. The three factors together explained 43.0 % of the variance in SB; SEE = 71.0 (model p < 0.001).
Cardiorespiratory fitness
Furthermore, univariate CRF was significantly associated with percentage of fat mass (β −0.8; 95 % CI: −1.0; (−0.5); p < 0.001), being during (0)/after (1) cancer treatment (β 7.4; 95 % CI: 2.4; 12.3; p = 0.004), fatigue (β 0.2; 95 % CI: 0.1; 0.3; p < 0.001), depressive symptoms (β −0.8; 95 % CI: −1.3; (−0.4); p = 0.001), and athletic competence (β 0.2; 95 % CI: 0.1; 0.2; p < 0.001) (Table 3). For the multiple regression analyses, also lower body muscle strength (β 0.0; 95 % CI: 0.0; 0.0; p = 0.055) was added as an independent variable.
The multiple linear regression analysis for CRF showed that fat mass (β −0.5; 95 % CI: −0.7; (−0.2); p < 0.001), being during/after treatment (β 3.9; 95 % CI: 0.3; 7.5; p = 0.035), fatigue (β 0.2; 95 % CI: 0.1; 0.3; p < 0.001), and beliefs of athletic competence (β 0.1; 95 % CI: 0.0; 0.1; p = 0.034) were significantly associated with CRF. Thus, fatigued children with increased fat mass and reduced beliefs in athletic competence and those during cancer treatment had the lowest CRF. These four factors explained 64.8 % of the variance in CRF; SEE = 5.7 (model p < 0.001).
Discussion
The present study shows that the CRF is low in the majority of children during and after cancer treatment when compared to healthy Dutch children. Furthermore, this is the first study performed in children with cancer that clearly demonstrates that decreased CRF is significantly associated with objectively assessed low PA and high SB. Children at risk for reduced PA had the highest percentage of fat mass, were older and fatigued, and were during childhood cancer treatment. Unexpectedly, treatment-related factors did not significantly influence activity behavior. These results indicate that intervention studies should focus on preventing or reducing fatigue and overweight, in order to improve PA behavior and ultimately increase CRF. The most sedentary children of the study were older and were during childhood cancer treatment, pointing out an important target population.
Our finding that older age and fatigue were significantly associated with reduced PA is in line with previous findings among healthy children [14, 39, 40]. In children with cancer, however, next to an older age and being fatigued, Hooke et al. (2011) also found that children who exhibit emotional dysfunction were more sedentary [14]. The latter could not be confirmed with our data. Psychological factors in our study did not show a clear association pattern with PA and SB. In univariate models, depressive symptoms showed a significant association with the two outcomes; however, in multiple regression models, this factor did not remain significant. This indicates that this association was weak or possibly mediated by other factors [41].
International recommendations for children advice 60 min of moderate-to-vigorous physical activity per day [29]. The current study showed that 20 % of the boys and 7 % of the girls, during or shortly after childhood cancer treatment, met the activity recommendations, which, however, is in line with the worrisome results of the normal Dutch population [42]. This indicates that only a small percentage of all children, with or without cancer, reach the international PA recommendation. Yet, related to the given cancer treatment and possible late complications and diseases, the impact of inactivity in children with cancer may be worse than in healthy children [22].
Despite positive attitudes towards PA [43], the current study showed that children with cancer were highly sedentary. In particular, the prolonged periods of SB are striking; i.e., the sitting periods of 20 min or more were approximately four times higher in this study population, compared to reported data of healthy children [44]. The activity counts per minute were also considered lower than those reported in healthy children [45]. We found a median cpm score of 127 (IQR 80–219 cpm) equally distributed among sex, whereas a meta-analysis among 20,871 healthy children reported that girls had a mean PA of 540 cpm (193 SD) and boys a mean PA of 642 cpm (226 SD) (45). However, such as for PA data, the SB data of the study among healthy children were obtained with a different accelerometer (Actigraph) using different cutoff points for activity intensities, decreasing comparability [45].
Strength and limitations of this study
The strength of this study is the number of included children; 60 is a relatively large population compared to patient numbers used in the four previous studies which reported PA accelerometer data (range 7 to 38 patients) [4, 5, 15, 16]. Furthermore, this study is the first in children with cancer to combine CRF data with activity data and to show associations between activity behavior and patient characteristics. Finally, most of the data were obtained during a visit to the hospital for study purposes, increasing quality of the measurements.
This study also had some limitations that should be noted. First, the cross-sectional design does not allow for the assessment of the causal relation between study outcomes and factors. Longitudinal data of the QLIM RCT will provide further information regarding the relation between increased PA and CRF and possible confounding or mediating factors.
Secondly, in this study, accelerometer data were obtained for a period of 4 days instead of 7. The memory capacity of the accelerometer did not allow assessment of PA by 15-s epoch for a length of 7 days. It was possible to use 15-s epochs when we limited the assessment period to 4 days. The use of a short epoch in children is important because children are known to perform short and intermitted actions [27]. Missing data of 3 days within the measurement week is a limitation. However, accelerometer data were obtained from Wednesdays until Saturdays, which are the most common days in the week in the Netherlands to participate in (team) sports during childhood.
Sport participation before diagnosis was questioned retrospectively. However, the time period at which they participated in sports was not specified. This led to unclear information. To increase validity of the data, we dichotomized sport participation before diagnosis (yes/no), however, thereby losing some valid information.
Finally, this study included children with any type of cancer, aged between 8–18 years, and children both during and within the first year after cancer treatment. Therefore, our study group was a heterogeneous one with potentially additional influencing factors. However, as a result of this heterogeneity, we now were able to say that children with any type of cancer had reduced CRF and PA levels.
In conclusion, the present study shows that CRF is low in children during and shortly after cancer treatment and that this low fitness is associated with reduced PA levels and increased SB across all cancer and treatment types. It revealed that older, more fatigued children who were during cancer treatment were the least active. Increased SB, in addition, was significantly associated with older age, more fatigue, and having a higher percentage of fat mass. This indicates that especially the fatigued, overweight, or obese adolescents with cancer, and those who are during cancer treatment, need to be informed about the health risks of a prolonged sedentary lifestyle and be advised on how to increase their PA level. In the QLIM RCT, we will assess the causal relation between CRF, PA, SB, fatigue, age, and treatment-related factors in children with cancer, to develop an optimal exercise intervention for this population, in order to increase PA and CRF to ultimately decrease chronic diseases and impaired HrQoL later in life.
Reference
De Caro E, Fioredda F, Calevo MG, Smeraldi A, Saitta M, Hanau G et al (2006) Exercise capacity in apparently healthy survivors of cancer. Arch Dis Child 91(1):47–51
Van Brussel M, Takken T, van der Net J, Engelbert RHH, Bierings MAGC, Schoenmakers M (2006) Physical function and fitness in long-term survivors of childhood leukaemia. Pediatr Rehabil 9(3):267–274
Järvelä LS, Niinikoski H, Lähteenmäki PM, Heinonen OJ, Kapanen J, Arola M et al (2010) Physical activity and fitness in adolescent and young adult long-term survivors of childhood acute lymphoblastic leukaemia. J Cancer Surviv 4(4):339–345
Tan SY, Poh BK, Chong HX, Ismail MN, Rahman J, Zarina AL et al (2013) Physical activity of pediatric patients with acute leukemia undergoing induction or consolidation chemotherapy. Leuk Res 37(1):14–20
Fuemmeler BF, Pendzich MK, Clark K, Lovelady C, Rosoff P, Blatt J et al (2013) Diet, physical activity, and body composition changes during the first year of treatment for childhood acute leukemia and lymphoma. J Pediatr Hematol Oncol 35(6):437–443
Rogers PC, Meacham LR, Oeffinger KC, Henry DW, Lange BJ (2005) Obesity in pediatric oncology. Pediatr Blood Cancer 45(7):881–891
Green JL, Knight SJ, Mccarthy M, de Luca CR (2013) Motor functioning during and following treatment with chemotherapy for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer 60:1261–1266
Sharkey A, Carey A, Heise C, Barber G (1993) Cardiac rehabilitation after cancer therapy in children and young adults. Am J Cardiol 71(16):1488–1490
Te Winkel ML, Pieters R, Hop WCJ, Roos JC, Bökkerink JPM, Leeuw J a, et al. Bone mineral density at diagnosis determines fracture rate in children with acute lymphoblastic leukemia treated according to the DCOG-ALL9 protocol. Bone. Elsevier Inc.; 2014;59:223–8.
Soares-Miranda L, Fiuza-Luces C, Lucia A (2012) Physical activity and recovery from hematologic malignancy. In: Mittelman SD, Berger NA (eds) Energy balance and hematologic malignancies. New York, Springer, pp 159–176
Mulder RL, Thönissen NM, van der Pal HJH, Bresser P, Hanselaar W, Koning CCE et al (2011) Pulmonary function impairment measured by pulmonary function tests in long-term survivors of childhood cancer. Thorax 66(12):1065–1071
Winter CC, Müller C, Hardes J, Boos J, Gosheger G, Rosenbaum D (2012) Pediatric patients with a malignant bone tumor: when does functional assessment make sense? Support Care Cancer 20(1):127–133
Odame I, Duckworth J, Talsma D, Beaumont L, Furlong W, Webber C et al (2006) Osteopenia, physical activity and health-related quality of life in survivors of brain tumors treated in childhood. Pediatr Blood Cancer 46(3):357–362
Hooke M, Garwick A, Gross C (2011) Fatigue and physical performance in children and adolescents receiving chemotherapy. Oncol Nurs Forum 38:649–657
Aznar S, Webster AL, San Juan AF, Chamorro-Viña C, Maté-Muñoz JL, Moral S et al (2006) Physical activity during treatment in children with leukemia: a pilot study. Appl Physiol Nutr Metab 31:407–413
Harz KJ, Müller HL, Waldeck E, Pudel V, Roth C (2003) Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab 88(11):5227–5231
Altenburg TM, Rotteveel J, Dunstan DW, Salmon J, Chinapaw MJM (2013) The effect of interrupting prolonged sitting time with short, hourly, moderate-intensity cycling bouts on cardiometabolic risk factors in healthy, young adults. J Appl Physiol 115(12):1751–1756
Tremblay M (2012) Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab 37(3):540–542
Courneya KS, Friedenreich CM (2011) Physical activity and cancer. Courneya KS, Friedenreich CM, editors. Springer-Verlag, Berlin Heidelberg
Lumb A (2014) Diabetes and exercise. Clin Med (Northfield Il) 14(6):673–676
Seron P, Lanas F, Pardo Hernandez H, Bonfill CX (2011) Exercise for people with high cardiovascular risk. Cochrane Database Syst Rev 8
Geenen MM, Cardous-ubbink MC, Kremer LCM, Heinen RC, Jaspers MWM, Koning CCE et al (2013) Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297(24):2705–2715
Gotte M, Kesting S, Winter C, Rosenbaum D, Boos J (2014) Comparison of self-reported physical activity in children and adolescents before and during cancer treatment. Pediatr Blood Cancer 61:1023–1028
Braam KI, van Dijk EM, Veening MA, Bierings MB, Merks JHM, Grootenhuis MA et al (2010) Design of the Quality of Life in Motion (QLIM) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of a combined physical exercise and psychosocial training program to improve physical fitness in children with cancer. BMC Cancer 10(1):624
Bongers BC, van Brussel M, Hulzebos EH, Takken T. Pediatric norms for cardiopulmonary exercise testing. second. ’s Hertogenbosch: Uitgeverij BOXPress; 2014.
Puyau M, Adolph A, Vohra F, Zaker I, Butte N (2004) Prediction of activity energy expenditure using accelerometers in children. Med Sci Sport Exerc 36(9):1625–1631
Edwardson CL, Gorely T (2010) Epoch length and its effect on physical activity intensity. Med Sci Sports Exerc 42(5):928–934
Colley RC, Tremblay MS (2011) Moderate and vigorous physical activity intensity cut-points for the Actical accelerometer. J Sports Sci 29(8):783–789
Janssen I, LeBlanc AG (2010) Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int J Behav Nutr Phys Act 7(1):40
Verloigne M, Ridgers N, Chin A Paw M, Teatske A, Bere E, Van Lippevelde W, et al. The UP4FUN Intervention Effect on Breaking Up Sedentary Time in 10- to 12-Year-Old Belgian Children: The ENERGY-Project. Pediatr Exerc Sci. 2014
A. Rijpstra. De TNO groeicalculator [Internet]. 2013. Available from: http://groeiweb.pgdata.nl/Groeiweboud/calculator.asp
Beenakker EA, van der Hoeven J, Fock J, Maurits N (2001) Reference values of maximum isometric muscle force obtained in 270 children aged 4–16 years by hand-held dynamometry. Neuromuscul Disord 11(5):441–446
Verschuren O, Ketelaar M, Takken T, Van Brussel M, Helders PJM, Gorter JW (2008) Reliability of hand-held dynamometry and functional strength tests for the lower extremity in children with cerebral palsy. Disabil Rehabil 30(18):1358–1366
Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL et al (2012) A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res 27(10):2208–16
Gordijn MS, Cremers EMP, Kaspers GJL, Gemke RJBJ (2011) Fatigue in children: reliability and validity of the Dutch PedsQLTM Multidimensional Fatigue Scale. Qual Life Res 20(7):1103–1108
Van Dongen-Melman JEWM, Koot HM, Verhulst FC (1993) Cross-cultural validation of Harter’s self-perception profile for children in a Dutch sample. Educ Psychol Meas 53(3):739–753
Finch A, Saylor CF, Edwards GL, McIntosh JA (1987) Children’s depression inventory: reliability over repeated administrations. J Clin Child Psychol 16(4):339–341
Van Dijk-Lokkart EM, Braam KI, Huisman J, Kaspers GJ, Takken T, Veening M a, et al. Factors influencing childhood cancer patients to participate in a combined physical and psychosocial intervention program: Quality of Life in Motion. Psychooncology. 2014 Oct 6;
Finne E, Bucksch J, Lampert T, Kolip P (2011) Age, puberty, body dissatisfaction, and physical activity decline in adolescents. Results of the German Health Interview and Examination Survey (KiGGS). Int J Behav Nutr Phys Act 8(1):119
Brodersen N, Steptoe A, Williamson S, Wardle J (2005) Sociodemographic, developmental, environmental, and psychological correlates of physical activity and sedentary behavior at age 11 to 12. Ann Behav Med 29(1):2–11
Gilliam MB, Madan-Swain A, Whelan K, Tucker DC, Demark-Wahnefried W, Schwebel DC (2013) Cognitive influences as mediators of family and peer support for pediatric cancer survivors’ physical activity. Psychooncology 22(6):1361–1368
Verloigne M, Van Lippevelde W, Maes L, Yıldırım M, Chinapaw M, Manios Y et al (2012) Levels of physical activity and sedentary time among 10- to 12-year-old boys and girls across 5 European countries using accelerometers: an observational study within the ENERGY-project. Int J Behav Nutr Phys Act 9:34
Gotte M, Kesting S, Winter C, Rosenbaum D, Boos J (2014) Experience of barriers and motivations for physical activities and exercise during treatment of pediatric patients with cancer. Pediatr Blood Cancer 61:1632–1637
Altenburg TM, de Niet M, Verloigne M, De Bourdeaudhuij I, Androutsos O, Manios Y et al (2014) Occurrence and duration of various operational definitions of sedentary bouts and cross-sectional associations with cardiometabolic health indicators: the ENERGY-project. Prev Med 71C:101–106
Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A (2012) Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA 307(7):704–712
Acknowledgment
We thank Teatske M. Altenburg from the Department of Public and Occupational Health, EMGO institute for health care research (VU University Medical Center, the Netherlands) for the advice on assessing sedentary behavior, particularly on assessing sitting behavior.
This study is part of the A-CaRe Program, www.a-care.org. The authors acknowledge the A-CaRe Clinical Research Group. The research is supported by the Alpe d'HuZes/KWF Fund; the research grant is bestowed upon the Dutch Cancer Society (grant number ALPE-VU 2009-4305), the RopaRun, and the VUmc Childhood Cancer Research Foundation (VONK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Eline van Dulmen–den Broeder and Margreet A. Veening contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Braam, K.I., van Dijk-Lokkart, E.M., Kaspers, G.J. et al. Cardiorespiratory fitness and physical activity in children with cancer. Support Care Cancer 24, 2259–2268 (2016). https://doi.org/10.1007/s00520-015-2993-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2993-1