Abstract
Purpose
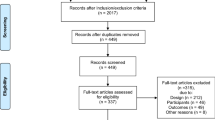
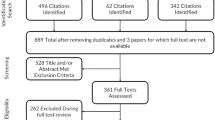
The process of assessing patient symptoms and functionality using patient-reported outcomes (PROs) and functional performance status (FPS) is an essential aspect of patient-centered oncology research and care. However, PRO and FPS measures are often employed separately or inconsistently combined. Thus, the purpose of this study was to conduct a systematic review of the level of association between PRO and FPS measures to determine their differential or combined utility.
Methods
A systematic search was conducted using five databases (1966 to February 2014) to identify studies that described an association between PRO and FPS. Studies were excluded if they were non-cancer specific, did not include adults aged 18 or older, or were review articles. Publications were selected for review by consensus among two authors, with a third author arbitrating as needed.
Results
A total of 18 studies met inclusion criteria. FPS was primarily assessed by clinicians using the ECOG Performance Status or Karnofsky Performance Status measures. PROs were captured using a variety of measures, with numerous domains assessed (e.g., pain, fatigue, and general health status). Concordance between PROs and FPS measures was widely variable, falling in the low to moderate range (0.09–0.72).
Conclusions
Despite consistency in the method of capture of PROs or FPS, domain capture varied considerably across reviewed studies. Irrespective of the method of capturing PROs or FPS, the quantified level of association between these two areas was moderate at best, providing evidence that FPS and PRO assessments offer unique information to assist clinicians in their decision-making.

Similar content being viewed by others
References
Kotronoulas G, Kearney N, Maguire R et al (2014) What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 32:1480–1501
Wilson IB, Cleary PD (1995) Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 273:59–65
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 5:649–655
Karnofsky DA, Burchenal JH (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM (ed) Evaluation of chemotherapeutic agents. Columbia University Press, New York, pp 199–205
Basch E, Jia X, Heller G et al (2009) Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 101:1624–1632
Basch E (2010) The missing voice of patients in drug-safety reporting. N Engl J Med 362:865–869
Basch E, Snyder C, McNiff K et al (2014) Patient-reported outcome performance measures in oncology. J Oncol Pract 10:209–211
Basch E (2012) Beyond the FDA PRO guidance: steps toward integrating meaningful patient-reported outcomes into regulatory trials and US drug labels. Value Health 15:401–403
Bruner DW, Hanisch LJ, Reeve BB et al (2011) Stakeholder perspectives on implementing the national cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Behav Med Pract Policy Res 1:110–122
Basch E, Abernethy AP, Mullins CD et al (2012) Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol 30:4249–4255
Judson TJ, Bennett AV, Rogak LJ et al (2013) Feasibility of long-term patient self-reporting of toxicities from home via the internet during routine chemotherapy. J Clin Oncol 31:2580–2585
Basch E (2014) New frontiers in patient-reported outcomes: adverse event reporting, comparative effectiveness, and quality assessment. Annu Rev Med 65:307–317
US Department of Health and Human Services (2009) Guidance for industry. Patient-reported outcome measures: Use in medical development to support labeling claims December 2009. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. Accessed 27 Jul 2015
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52:377–384
Bergman B, Sullivan M, Sörenson S (1991) Quality of life during chemotherapy for small cell lung cancer. I. An evaluation with generic health measures. Acta Oncol 30:947–957
Butt Z, Lai JS, Rao D et al (2013) Measurement of fatigue in cancer, stroke, and HIV using the functional assessment of chronic illness therapy - fatigue (FACIT-F) scale. J Psychosom Res 74:64–68
Carter GC, Liepa AM, Zimmermann AH, Morschhauser F (2008) Validation of the functional assessment of cancer therapy - lymphoma (FACT-LYM) in patients with relapsed/refractory mantle cell lymphoma. Paper presented at: 50th American society of hematology annual meeting and exposition; December 7, San Francisco, CA
Cella D, Beaumont JL, Webster KA, Lai JS, Elting L (2006) Measuring the concerns of cancer patients with low platelet counts: the functional assessment of cancer therapy—thrombocytopenia (FACT-Th) questionnaire. Support Care Cancer 14:1220–1231
Colwell HH, Mathias SD, Turner MP et al (2010) Psychometric evaluation of the FACT colorectal cancer symptom index (FCSI-9): reliability, validity, responsiveness, and clinical meaningfulness. Oncologist 15:308–316
Jones D, Zhao F, Fisch MJ et al (2014) The validity and utility of the MD Anderson symptom inventory in patients with prostate cancer: evidence from the symptom outcomes and practice patterns (SOAPP) data from the eastern cooperative oncology group. Clin Genitourin Cancer 12:41–49
Rothrock NE, Jensen SE, Beaumont JL et al (2013) Development and initial validation of the NCCN/FACT symptom index for advanced kidney cancer. Value Health 16:789–796
Walker MS, Hasan M, Yim YM et al (2011) Retrospective study of the effect of disease progression on patient reported outcomes in HER-2 negative metastatic breast cancer patients. Health Qual Life Outcomes 9:46
Yost KJ, Sorensen MV, Hahn EA et al (2005) Using multiple anchor- and distribution-based estimates to evaluate clinically meaningful change on the functional assessment of cancer therapy-biologic response modifiers (FACT-BRM) instrument. Value Health 8:117–127
Yount S, Cella D, Webster K et al (2002) Assessment of patient-reported clinical outcome in pancreatic and other Hepatobiliary cancers: the FACT Hepatobiliary symptom index. J Pain Symptom Manag 24:32–44
Bock M, Moore D, Hwang J et al (2012) The impact of an electronic health questionnaire on symptom management and behavior reporting for breast cancer survivors. Breast Cancer Res Treat 134:1327–1335
Robinson DW Jr, Eisenberg DF, Cella D et al (2008) The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. J Support Oncol 6:283–290
Velanovich V, Wollner I (2011) Quality of life and performance status in patients with pancreatic and periampullary tumors. Int J Clin Oncol 16:401–407
Anderson F, Downing GM, Hill J, Casorso L, Lerch N (1996) Palliative performance scale (PPS): a new tool. J Palliat Care 12:5–11
Kamal AH, Bull J, Stinson CS, Blue DL, Abernethy AP (2013) Conformance with supportive care quality measures is associated with better quality of life in patients with cancer receiving palliative care. J Oncol Pract 9:e73–76
Butt Z, Peipert J, Webster K, Chen C, Cella D (2013) General population norms for the functional assessment of cancer therapy-kidney symptom index (FKSI). Cancer 119:429–437
List MA, Ritter-Sterr C, Lansky SB (1990) A performance status scale for head and neck cancer patients. Cancer 66:564–569
Karnell LH, Funk GF, Tomblin JB, Hoffman HT (1999) Quality of life measurements of speech in the head and neck cancer patient population. Head Neck 21:229–238
Singh JA, Satele D, Pattabasavaiah S, Buckner JC, Sloan JA (2014) Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales. Health Qual Life Outcomes 12:187
Ware JE, Snow KK, Kosinski M, Gandek B (1993) SF-36 health survey: manual and interpretation guide. Health Institute, New England Medical Centre, Boston, MA
Dolan P (1997) Modeling valuations for EuroQol health states. Med Care 35:1095–1108
Cella DF, Tulsky DS, Gray G et al (1993) The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Webster K, Cella D, Yost K (2003) The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 1:79
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Cleeland CS, Mendoza TR, Wang XS et al (2000) Assessing symptom distress in cancer patients: the M.D. Anderson symptom inventory. Cancer 89:1634–1646
Daut RL, Cleeland CS (1982) The prevalence and severity of pain in cancer. Cancer 50:1913–1918
Daut RL, Cleeland CS, Flanery RC (1983) Development of the Wisconsin brief pain questionnaire to assess pain in cancer and other diseases. Pain 17:197–210
Cella D, Riley W, Stone A et al (2010) The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 63:1179–1194
Xiao C, Polomano R, Bruner DW (2013) Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs 36:E1–E16
Ryan S, Atkinson TM, Bennett AV et al. (2013) Concordance between symptomatic adverse event ratings by clinicians and patients: a systematic review. Paper presented at: 20th annual meeting for the international society for quality of life research; October 10, Miami, FL.
Basch E, Bennett A, Pietanza MC (2011) Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J Natl Cancer Inst 103:1808–1810
National Cancer Institute, National Institutes of Health, US Department of Health and Human Services (2010) Common terminology criteria for adverse events (CTCAE) Version 4.0. Published May 28, 2009; Revised Version 4.03 June 14, 2010 Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 7 Jun 2015
Basch E, Reeve BB, Mitchell SA et al (2014) Development of the national cancer institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 106(9), 21
Hay JL, Atkinson TM, Reeve BB et al (2014) Cognitive interviewing of the US national cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res 23:257–269
Bruner DW, Movsas B, Basch E (2012) Capturing the patient perspective: patient-reported outcomes as clinical trial endpoints. Am Soc Clin Oncol Educ Book 2012:139–144
Blaauwbroek R, Bouma MJ, Tuinier W et al (2009) The effect of exercise counselling with feedback from a pedometer on fatigue in adult survivors of childhood cancer: a pilot study. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 17:1041–1048
Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR (2007) Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol Off J Am Soc Clin Oncol 25:2352–2359
Bourke L, Homer KE, Thaha MA et al (2013) Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev 9:Cd010192
Acknowledgments
This project was supported by a National Institutes of Health Research Training Grant (T32 CA009461-25); as well as a National Institutes of Health Support Grant (P30 CA08748-48), which provides partial support for the Behavioral Research Methods Core Facility used in conducting this investigation. A portion of these results were presented at the 2015 Meeting of the Society of Behavioral Medicine in San Antonio, TX.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atkinson, T.M., Andreotti, C.F., Roberts, K.E. et al. The level of association between functional performance status measures and patient-reported outcomes in cancer patients: a systematic review. Support Care Cancer 23, 3645–3652 (2015). https://doi.org/10.1007/s00520-015-2923-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2923-2