Abstract
Purpose
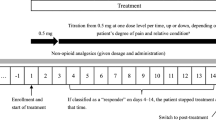
The recommended dosing interval for transdermal fentanyl is every 72 h. However, some patients will have “end-of-dose failure,” which may be seen as an increase of episodes of severe pain flares at the third day after application of the patch. A new once-a-day fentanyl patch was developed in Japan since 2010. This study aimed to assess the efficacy of the once-a-day fentanyl citrate patch for patients with cancer-related pain receiving the 72-h transdermal fentanyl not lasting 72 h.
Methods
We performed a cross-sectional retrospective analysis of 445 inpatients with the 72-h transdermal fentanyl at Higashi Sapporo Hospital. We could switch to the once-a-day fentanyl citrate patch if patients reported inadequate pain relief beyond 48 h after application of the 72-h transdermal fentanyl. Patients recorded baseline scores for background pain intensity (PI) and the frequency of use of daily rescue medication for breakthrough cancer pain (BTcP).
Results
Of all patients, 10.1 % showed the increase in PI of 30 % or more baseline PI on the third day after application of the 72-h transdermal fentanyl. Of patients, 84.4 % were converted from equivalent dose of the 72-h transdermal fentanyl to the once-a-day fentanyl citrate patch. On the third day after switching, 60.5 % of patients showed a reduction of more than 30 % from baseline PI. Switching to the once-a-day fentanyl citrate patch significantly reduced the mean frequency of daily rescue dose for BTcP.
Conclusions
A once-a-day fentanyl citrate patch provided stable pain control. Its use may be considered as the dominant strategy for patients receiving a 72-h transdermal fentanyl not lasting 72 h.



Similar content being viewed by others
References
Muijsers RBR, Wagstaff AJ (2001) Transdermal fentanyl: an update review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs 61(15):2289–2307
Payne R (1998) Factors influencing quality of life in cancer patients; the role of transdermal fentanyl in the management of pain. Semin Oncol 25(3 Suppl 7):47–53
Marier JF, Lor M, Potvin D et al (2006) Pharmacokinetics, tolerability and performances of a novel matrix transdermal delivery system of fentanyl relative to the commercially available reservoir formulation in healthy subjects. J Clin Pharmacol 46(6):642–653
US Food and Drug Administration. Information for healthcare professionals: Fentanyl Transdermal System (marketed as Duragesic and generics) (2007) http://www.accessdata.fda.gov/drugsatfda_docs/…/19813s039lbl.pdf Accessed 3 May 2015
Zeppetella G (2011) Breakthrough pain in cancer patients. Clin Oncol 23:393–398
Takakuwa O, Oguri T, Maeno K et al (2013) Analgesic effect of switching from oral opioids to a once-a-day fentanyl citrate transdermal patch in patients with lung cancer. Am J Hosp Palliat Care 30(7):726–729
Caraceni A, Bertetto O, Labianca R et al (2012) Episodic (breakthrough) pain prevalence in a population of cancer pain patients. Comparison of clinical diagnoses with the QUDEI—Italian questionnaire for intense episodic pain. J Pain Symptom Manage 43(5):833–841
Farrar JT, Young JP Jr, LaMoreaux L et al (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94:149–158
Hall LM, O’lenic K (2012) Treatment strategies to overcome and end-of-dose failure with oral and transdermal opioids. J Pharm Pract 25:503–509
FENTOS® TAPE (once-a-day fentanyl citrate patch) [package insert (in Japanese)] (2014) www.info.pmda.go.jp/…/650034_8219701S1025_1_09.pdf Accessed 3 May 2015
Uchida E, Miyazaki T, Namiki A et al (2010) Pharmacokinetic studies of the transdermal patch containing fentanyl citrate (FT-290) in patients with cancer pain. Journal of clinical therapeutics and medicines 26:335–351
Donner B, Zenz M, Strumpf M et al (1998) Long-term treatment of cancer pain with transdermal fentanyl. J Pain Symptom Manage 15:168–175
Grond S, Zech D, Lehmann KA et al (1997) Transdermal fentanyl in the long term treatment of cancer pain: a prospective study of 50 patients with advanced cancer of the gastrointestinal tract or the head and neck region. Pain 69:191–198
Kim DY, Song HS, Ahn JS et al (2010) The dosing frequency of sustained-release opioids and the prevalence of end-of-dose failure in cancer pain control: a Korean multicenter study. Support Care Cancer 19(2):297–301
Kokubun H, Matoba M, Hoka S et al. (2007) Relationship between serum fentanyl concentration and transdermal fentanyl dosage, and intra-individual variability in fentanyl concentration after application of fentanyl patches in patients with cancer pain. J Pharm Health Care Sci 33(3):200–205
Davis AN, Dickman A, Reid C, Science Committee of the Association for Palliative Medicine of Great Britain and Ireland et al (2009) The management of cancer-related breakthrough pain; recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain 13:331–338
Haugen DF, Hjermstad MJ, Hagen N, European Palliative Care Research Collaborative (EPCRC) et al (2010) Assessment and classification of breakthrough cancer pain: a systematic literature review. Pain 149:476–482
National Comprehensive Cancer Network (Version 2. 2014). NCCN Clinical Practice Guidelines in Oncology, adult cancer pain. http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf Accessed 3 May 2015
Caraceni A, Hanks G, Kaasa S, The European Palliative Care Research Collaborative (EPCRC), on behalf of the European Association for Palliative Care (EAPC) et al (2012) Use of opioid analgesic in the treatment of the cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 13:e58–e68
Caraceni A, Davies A, Poulain P et al (2013) Guidelines for the management of breakthrough pain in patients with cancer. J Natl Compr Canc Netw 11:S29–S36
European Oncology Nursing Society guidelines (2013): breakthrough cancer pain guidelines http://www.cancernurse.eu/documents/EONSBreakthroughCancerPainGuidelines.pdf Accessed 3 May 2015
Mercadante S (2011) Managing breakthrough pain. Curr Pain Headache Rep 15:244–249
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was accepted as a poster at the Multinational Association on Supportive Care in Cancer (MASCC)/ISOO 2013 International Symposium in Berlin, Germany, June 27–29, 2013.
Rights and permissions
About this article
Cite this article
Koike, K., Terui, T., Nagasako, T. et al. A new once-a-day fentanyl citrate patch (Fentos® Tape) could be a new treatment option in patients with end-of-dose failure using a 72-h transdermal fentanyl matrix patch. Support Care Cancer 24, 1053–1059 (2016). https://doi.org/10.1007/s00520-015-2880-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2880-9