Abstract
Purpose
There are concerns regarding potential negative effects of prophylactic treatment of epidermal growth factor receptor (EGFR)-inhibitor-related rashes on metastatic colorectal cancer (mCRC) outcomes. We aimed to characterize treatment patterns of EGFR-inhibitor-induced rashes and evaluate prophylactic versus reactive approaches to rash management in relation to overall survival (OS).
Methods
Patients diagnosed with KRAS wild-type mCRC from July 2010 to June 2012 in British Columbia and prescribed cetuximab or panitumumab were reviewed to describe patterns of use of oral antibiotics and steroid creams. Using Cox regression, the relationship between prophylactic versus reactive rash management and OS was characterized.
Results
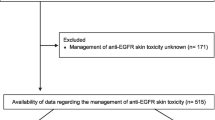
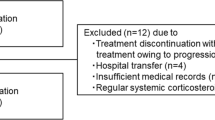
A total 119 patients were analyzed: median age was 63 years, 61 % were male, 34 % received cetuximab, 66 % received panitumumab, and median number of EGFR inhibitor treatment was nine cycles. Rash occurred in >90 % of patients, and reactive was favored over prophylactic treatment (66 vs. 34 %). Older patients and those with Eastern Cooperative Oncology Group (ECOG) performance status 0/1 were more likely to receive prophylactic creams (44 vs. 20 % for age <60, p = 0.01) and oral antibiotics (62 vs. 12 % for ECOG ≥2, p = 0.01), respectively. Median OS was 7.0 months. The number of treatment cycles and OS were similar in both prophylactic and reactive groups (both p > 0.05). In Cox regression, ECOG >2 correlated with worse survival (hazard ratio (HR) 22.01, 95 % confidence interval (CI) 5.25–92.30, p < 0.01). However, survival outcomes were similar between patients prescribed antibiotics prophylactically versus reactively (HR = 1.10, 95 % CI 0.43–2.80, p = 0.85), and steroid creams prophylactically versus reactively (HR = 2.00, 95 % CI 0.58–6.92, p = 0.27).
Conclusion
Prophylactic treatment of EGFR-inhibitor-related rashes is associated with similar outcomes compared to reactive rash treatment in mCRC.
Similar content being viewed by others
References
Li T, Perez-Soler R (2009) Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol 4:107
Jatoi A, Green EM, Rowland KM Jr et al (2009) Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in north central cancer treatment group study N0147. Oncology 77:120
Jacot W, Bessis D, Jorda E et al (2004) Acneiform eruption induced by epidermal growth factor receptor inhibitors in patients with solid tumours. Br J Dermatol 151:238
Busam KJ, Capodieci P, Motzer R et al (2001) Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 144:1169
Wierzbicki R, Jonker DJ, Moore MJ et al (2011) A phase II, multicenter study of cetuximab monotherapy in patients with refractory, metastatic colorectal carcinoma with absent epidermal growth factor receptor immunostaining. Invest New Drugs 29:170
Jatoi A, Nguyen PL (2008) Do patients die from rashes from epidermal growth factor receptor inhibitors? A systematic review to help counsel patients about holding therapy. Oncologist 13:1201–1204
Wagner LL, Lacouture ME (2007) Dermatologic toxicities associated with EGFR inhibitors: the clinical psychologist’s perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncology 21(suppl 5):34–36
Peréz-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol 23:5235–5246
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and Cetuximab plus Irinotecan in Irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:341
Agero AL, Dusza SW, Benvenuto-Andrade C et al (2006) Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 55:657
Perez-Soler R (2006) Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 8(suppl 1):S7
Kris M, Natale RB, Herbst RS et al (2003) Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: A randomized trial. JAMA 290:2149–2158
Lacouture ME, Mitchell EP, Piperdi B et al (2010) Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 28:1351–1352
Scope A, Agero AL, Dusza SW et al (2007) Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol 25:5390
Jatoi A, Rowland K, Sloan JA et al (2008) Tetracycline to prevent epidermal growth factor receptor inhibitor-induced skin rashes: results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB). Cancer 113:847
Saltz L, Kjes MS, Abbruzzese J et al (2003) The presence and intensity of the cetuximab-induced acne-like rash predicts increased survival in studies across multiple malignancies. Proc Am Soc Clin Oncol 22:204 (abstr 817)
Melosky B, Burkes R, Rayson D et al (2009) Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol 16:16–26
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 5:649–655
Peeters M, Siena S, Van Cutsem E et al (2009) Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving Panitumumab monotherapy. Cancer 115:1544
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label, phase 3 clinical trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
Saltz LB, Meropol NJ, Loehrer PJ Sr et al (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208
Perez-Soler R, Chachoua A, Hammond LA et al (2004) Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol 22:3238–3247
Acknowledgments
Authors would like to gratefully acknowledge the British Columbia Cancer Foundation and the Canadian Cancer Society Research Institute for their support in making this research possible.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by British Columbia Cancer Foundation and the Canadian Cancer Society Research Institute.
Prior submission
There are no prior submissions to other journals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dascalu, B., Kennecke, H.F., Lim, H.J. et al. Prophylactic versus reactive treatment of acneiform skin rashes from epidermal growth factor receptor inhibitors in metastatic colorectal cancer. Support Care Cancer 24, 799–805 (2016). https://doi.org/10.1007/s00520-015-2846-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2846-y