Abstract
Purpose
The use of serotonin type 3 (5-HT3) receptor antagonists (RAs) in the prevention of nausea and vomiting caused by emetogenic chemotherapy is part of a comprehensive management strategy for patients undergoing chemotherapy. Electrocardiographic effects have been reported in patients after intravenous administration of 5-HT3 RAs. The present study investigated the electrocardiogram (ECG) profile of the 5-HT3 RA palonosetron following International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) E14 Guidelines.
Methods
A total of 221 healthy subjects (101 females, 120 males) were randomized in this phase I, double-blind, double-dummy, parallel group study and assigned to one of five treatments: placebo, palonosetron (0.25, 0.75, or 2.25 mg), or moxifloxacin (400 mg). ECGs were recorded for 24 h pre-dosing until 48 h post-dose. The primary endpoint was the placebo time-matched and baseline-subtracted individual QTc interval prolongation (ΔΔQTcI).
Results
The QTc interval was not prolonged after administration of palonosetron (ΔΔQTcI upper confidence interval was <10 ms for all time points in all palonosetron treatment groups). Assay sensitivity was confirmed with the expected change in the QTc interval after administration of the positive control moxifloxacin.
Conclusions
Palonosetron, even at supratherapeutic doses, has no effect on cardiac repolarization as measured by the QTc interval in a validated controlled clinical trial.
Similar content being viewed by others
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a distressing and debilitating side effect of chemotherapeutic regimens that can profoundly affect quality of life [1, 2]. Different agents, including metoclopramide and corticosteroids, have been historically used to help patients control side effects but with limited efficacy [3]. At present, 5-HT3 receptor antagonists (RAs) are considered to be a central part of an effective prophylactic regimen against CINV. Combination regimens including 5-HT3 RAs are currently recommended to prevent nausea and vomiting induced by highly and moderately emetogenic chemotherapy [4–6].
The 5-HT3 RAs dolasetron, ondansetron, and granisetron are also indicated for the prevention of postoperative nausea and vomiting (PONV) [7]; 5-HT3 RAs are administered at the end of surgery conducted under total anesthesia. Indeed, the combination of a 5-HT3 RA with dexamethasone and droperidol is sometimes used in high-risk PONV patients [7].
Antiarrhythmic drugs (e.g., amiodarone, quinidine, and sotalol) but also noncardiac medications from a variety of therapeutic classes, including 5-HT3 RAs, have been associated with prolongation of cardiac repolarization as measured by the corrected QT (QTc) interval on the electrocardiogram (ECG). These drugs have the potential to influence the Ikr cardiac potassium channel, thereby resulting in increased repolarization time [8–10]. To date, the primary clinical implication of repolarization changes is the increased risk of possible fatal cardiac events, i.e., torsades de pointes [10]. Therefore, there is a regulatory mandate to define the potential of QTc interval prolongation induced by noncardiac drugs. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) E14 Guidelines, effective from November 2005 [11], was developed to help drive adequate trials in healthy volunteers in order to ascertain the effects on QTc interval prolongation of new nonantiarrhythmic medications.
Most of the cases of QTc interval prolongation and related transient arrhythmias described in the literature have been observed in the PONV setting with either dolasetron [12–15] or ondansetron [16–18]. The addition of certain risk factors (e.g., congenital long QT syndrome, hypokalemia, or female gender) have been suggested as potential inducers for torsades de pointes [10]. The prescribing information for both dolasetron and ondansetron carries a cardiovascular warning. A more recent study in healthy volunteers, designed on the basis of the E14 guidance, found that under controlled conditions, both droperidol and ondansetron, either alone or in combination, induced marked QTc interval prolongation [19]. However, the combination of both drugs did not significantly increase QTc interval prolongation compared to droperidol alone.
Palonosetron is a unique 5-HT3 RA that is characterized by a half-life of about 40 h compared to the first-generation 5-HT3 RAs such as ondansetron, dolasetron, and granisetron (4–9 h). The present study investigated the effects of single increasing intravenous (IV) doses (0.25 mg, 0.75, and supratherapeutic 2.25 mg) of palonosetron on the ECG profile in healthy volunteers compared to placebo and a positive control, namely oral moxifloxacin (400 mg). The study was designed, conducted, analyzed, and interpreted in accordance with the recommendations of the ICH E14 Guidelines as a “thorough QT/QTc study” [11].
Methods
This was a phase I, randomized, single-dose, double-blind, double-dummy, parallel group study to investigate the effects of clinical and supratherapeutic doses of palonosetron on the ECG profile compared to placebo. Moxifloxacin (Avelox, Bayer, film-coated capsules 400 mg) was used as a positive control to assess assay sensitivity [20]. Doses of palonosetron (Aloxi, Helsinn HealthCare, 0.25 and 0.75 mg/5 ml) were 0.25, 0.75, and 2.25 mg, which is ninefold higher than the US and European approved dose for CINV prophylaxis. The study was conducted at a single site (Swiss Pharma Contract Ltd., Allschwil, Switzerland) in accordance with the Swiss Federal Law on Medicine and Medical Devices, and approved by the local Ethics Committee.
Subjects were healthy volunteers between 18 and 65 years of age, either male or female, with a body weight within 20 % of ideal weight for height, frame, and age according to the 1983 Metropolitan Height/Weight Tables (Society of Actuaries and Association of Life Insurance Medical Directors of America Metropolitan Height and Weight Tables; Statistical Bulletin Metropolitan Life Insurance Company, Warwick R.I. Jan-Jun 1983; 2–9). All subjects provided written informed consent prior to the study. Specific exclusion criteria focusing on cardiac health performance at baseline were as follows: any type of significant cardiovascular disorder or family history of sudden cardiac death at age <50 years; any condition known to increase the possibility of QT interval prolongation; a relevant screening or baseline 12-lead ECG interval abnormality (i.e., PR interval >220 ms, QRS interval >120 ms, QT interval >450 ms).
Subjects were screened (ECG, laboratory values, medical history, and physical examination) prior to randomization.
A total of 230 healthy subjects were planned for inclusion in the study, accounting for an estimate of 5 % for dropouts and/or nonevaluable ECGs. The sample size was calculated based on the assumption of a difference of 5 ms between the individual corrected QT interval (QTcI) change from baseline of each dose of palonosetron and the QTcI change from baseline of placebo. For a two-sided test of difference, using a α = 0.05 and β = 0.2 (i.e., power 80 %), with a standard deviation of 8 ms, the sample size was estimated to be 42 subjects per treatment group. After screening, each eligible subject was assigned to a sequential subject number when the digital ECG recording started. Numbers were attributed according to the study entry. Five treatment groups were assigned: (i) placebo IV + placebo oral; (ii) palonosetron 0.25 mg IV + placebo oral; (iii) palonosetron 0.75 mg IV + placebo oral; (iv) palonosetron 2.25 mg IV + placebo oral; and (v) placebo IV + moxifloxacin 400 mg oral. To ensure an equal allocation of male and female subjects to the five treatments, two separate randomization lists (one for males and one for females) were used. Randomization lists were prepared at the Biometrics Department of Swiss Pharma Contract LTD, 4123 Allschwil, Switzerland, by a professional not involved in the study. One sealed copy was provided to Fischer Clinical Services AG, CH-4123 Allschwil, for packaging. The Investigator was provided with Code Breakers containing the treatment allocations, to be opened only in case of a medical emergency.
Three digital 12-lead ECGs (approximately 1 min apart) were recorded (Mortara Instrument ELI 250 12-lead digital recorder) serially for 24 h prior to dosing (at −23 h 45 min, −23 h 30 min, −23 h, −22 h, −20 h, −18 h, −16 h, −14 h, −12 h, −10 h, −8 h, and at 0 h) and through to 48 h post-dose at 18 time points (Table 1), starting 15 min post-administration. All ECGs were analyzed by a centralized ECG laboratory (eResearch Technology, Philadelphia, PA, USA) with interval measurements conducted by a high-resolution manual on-screen caliper method with annotations to minimize inter-reader variability. The central ECG laboratory was fully blinded as per E14 recommendations.
Blood samples were drawn by venous puncture immediately before study drug administration at 0.25, 0.5, 1, 2, 4, 8, 12, 24, 36, and 48 h post-dose. Use of an indwelling catheter was allowed at the discretion of the study staff. Plasma was frozen (−20 °C) and stored for analysis of study drug concentration by validated LC-MS/MS methods to evaluate any PK/PD relationship between plasma concentrations of palonosetron after intravenous administration and ECG QT and corrected QT intervals (Clinical Research Services, Mannheim GmbH, Department of Bioanalytics, Richard-Wagner-Straße 20, 67269 Grünstadt, Germany). A linear mixed effects model was calculated as the delta QT (∆QT) or delta QTc (∆QTc) versus the plasma concentration (as a fixed effect) with subject included in the model as a random effect.
The model is as follows: ΔQTc = α + β*(plasma concentration) + γ*(subject effect), where Δ is the change from baseline (calculated as a time-match difference), α is the intercept, β is the slope of the plasma concentration, and γ is the subject random effects parameter. If the p value of the slope for plasma concentration (β in the above model) is less than 0.05, then a linear effect of ∆QTc would be declared.
ICH E14 Guideline principles
The threshold of regulatory concern for QT/QTc interval prolongation is considered to be around 5 ms. This is the basis for the choice of moxifloxacin as the positive control, as it is known to have an effect on QT/QTc of around the threshold value. A negative “thorough QT/QTc study” is one in which the upper bound of the 95 % one-sided confidence interval for the largest time-matched mean effect of the drug on the QTc interval excludes 10 ms. This definition is chosen to provide a reasonable assurance that the mean effect on the QT/QTc interval is not greater than around 5 ms.
Time-matched analysis of QTc interval
The primary QT to QTc correction formula was determined for each subject by iterating the QT-RR relationship using the baseline ECGs to find an estimate for the exponent such that the slope of this relationship is closest to “0”. This is called the QTcI (I for individually determined QT correction, which is considered the most accurate method to correct QT for heart rate [HR]).
Consistent with ICH E14 Guidelines, the primary analysis was a time-matched analysis of the QTcI in which each time point on-treatment was compared with baseline value for the corresponding time point. This comparison represents the change of QTcI interval from baseline (delta QTcI [∆QTcI]) at each time point for each treatment arm. QTcI is the individually determined QT correction, and the goal is to find β such that QTcI is a constant, where QTcI = QT / (RR)β. This implies loge (QTcI) = loge (QT) − β × loge (RR). Because loge (QTcI) is a constant, one can re-write this equation as loge (QT) = α + β × loge (RR). Therefore, the exponent estimate can be obtained using regression analysis on log-transformed data based on the least squares approach.
After computation of the ΔQTcI for each time point, the mean placebo change from baseline at the corresponding time point was subtracted, generating a placebo-corrected change from baseline (delta-delta QTcI [ΔΔQTcI]). The primary parameter of interest in this study was the largest difference and the upper confidence interval (CI) bound of ΔΔQTcI. The hypothesis of QTcI prolongation of any dose of palonosetron was rejected if the upper limit of the one-sided 95 % CI for the ΔΔQTcI was less than 10 ms.
Other assessments
In addition to ΔΔQTcI (primary variable), other QTc correction formulae were considered secondary, including QTcFridericia (QTcF) and QTcBazett (QTcB). A time-averaged analysis, comparing the mean and maximum values on day 1 and day 1 + 2 after treatment with the corresponding value at baseline, was assessed for all ECG interval parameters (QT, QTcB, QTcF, QTcI, HR, PR, and QRS). In addition, change from baseline for ECG morphology was assessed. Concentration-QTcF relationship was assessed according to the recommendation of Garnett et al. [21].
Results
A total of 230 subjects were planned for inclusion in the study: 239 subjects were enrolled into the study, and 18 did not receive the treatment. Therefore, 221 subjects (101 females, 120 males) were included in the safety population analysis after receiving placebo or palonosetron (0.25, 0.75, or 2.25 mg) or moxifloxacin (400 mg). Mean age was 41.4 years, and the majority of subjects were Caucasian (211 out of 221). Baseline characteristics were similar across all treatment groups with no significant demographic differences.
Placebo-corrected time-matched analysis ΔΔQTcI
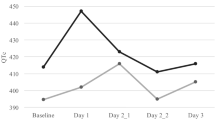
The upper boundary of the one-sided 95 % CI for all time-matched ΔΔQTcI time points for all three palonosetron doses and moxifloxacin are shown in Table 1. As shown, the upper bound of the one-sided 95 % ANOVA of the ΔΔ analysis was less than 10 ms for all time points across all palonosetron treatment groups, and the moxifloxacin upper bound exceeded 10 ms at five consecutive time points. In addition, the lower bound of the 95 % CI was >5 ms at three time points for the moxifloxacin group (data not shown), thus confirming that assay sensitivity was met, and therefore, a lack of evidence of QTc prolongation for all doses of palonosetron was validated. The mean treatment effect (change in QTcI) at each time point with two-sided 90 % CI to match the one-sided upper 95 % confidence bounds is shown in Fig. 1 for the placebo-corrected treatment effect.
The analyses were to be repeated by gender should an interaction of gender by treatment appear in the time-matched analysis. As the interaction treatment by gender term was not significant, this gender stratified analysis was not done.
ECG parameters and outlier analysis
The mean changes observed in HR, PR, and QRS durations, as well as QTc duration using all three correction formulae, were not clinically significant. Likewise, no outliers were found for HR, PR, and QRS durations.
An exploratory outlier analysis (e.g., new absolute QTcI >500 or >480 or >450 ms, or a change from baseline of 30–60 or >60 ms) was performed to look for QTcI changes in the palonosetron groups compared to placebo. No cases of specific outliers (new QTcI >500-ms change, or >480 or >450 ms, or >60-ms change, or abnormal U waves) were identified at any time point. Only one outlier for QTcI was observed in the 2.25 mg palonosetron dose group. This subject showed a 30–60 ms change from baseline, which was judged to likely be a case of spontaneous variability.
In addition, no differences in the placebo versus palonosetron dose groups for HR, PR, or QRS interval durations were observed. Furthermore, no negative (inverted) T waves or abnormal U waves were observed.
PK-PD relationship with QT variation
A PK/PD relationship between plasma concentrations of palonosetron after IV administration and ECG QT and corrected QT intervals was also explored. There was no statistical or experimental evidence to demonstrate any potential relationship for corrected QT interval or QT intervals with plasma palonosetron concentrations after single-dose administration of IV palonosetron, including supratherapeutic doses up to 2.25 mg which are ninefold greater than the currently marketed 0.25 mg IV dose (Fig. 2).
Safety
Overall, palonosetron at any dose was well tolerated in all subjects; no serious events or cardiac adverse events were reported.
A total of 125 adverse events (AEs) were reported, 98 of which were treatment-emergent (TEAEs). The number of TEAEs was similar across the treatment groups: 21 were reported in the placebo group; 15, 23, and 20 TEAEs were reported in the palonosetron 0.25 mg, palonosetron 0.75 mg, and palonosetron 2.25 mg groups, respectively; 19 TEAEs were reported in the moxifloxacin positive-control group.
Among the 98 TEAEs, 65 were of mild intensity, affecting mainly the nervous (N = 25, e.g., 24 were headache) and gastrointestinal (GI) systems (N = 16, mainly constipation and abdominal pain). Another ten mild TEAEs were coded as infections/infestations (eight were nasopharyngitis). Only one TEAE was classified as a cardiac disorder (palpitation). This mild and intermittent event was reported in a subject assigned to the placebo group, 6 h post-dose, and lasted approximately 8 h.
Thirty-two TEAEs were of moderate intensity, mostly affecting the GI (N = 11, e.g., constipation, dyspepsia, and flatulence) and nervous systems (N = 8, e.g., headache). Another eight were musculoskeletal disorders (e.g., backpain).
Only one TEAE (headache) was of severe intensity and was reported by one subject in the palonosetron 0.75 mg group.
Forty-eight of the 98 TEAEs occurred in 38 subjects and were judged to be “at least possibly” related to the study drugs: 11 out of 21 in the placebo group; 2 out of 15 in the palonosetron 0.25 mg group; 9 out of 23 in the palonosetron 0.75 mg group; 14 out of 15 in the palonosetron 2.25 mg group; and 12 out of 19 in the moxifloxacin treatment group. These events were mostly nervous system or GI disorders and generally known to be associated with palonosetron use (e.g., headache and constipation).
Discussion and conclusions
The major finding of this study is that palonosetron did not show significant effects on cardiac repolarization, as measured by the QTc interval in an ICH E14 compliant, moxifloxacin-controlled clinical trial. There were also no effects on HR, atrioventricular conduction (PR interval), depolarization (QRS interval duration), or wave morphology. In addition, there was no evidence of any relationship between palonosetron concentration and QT/QTc parameters over a wide concentration range. This study is also in agreement with recently published data in cancer patients, where palonosetron did not show changes in heart rate-corrected QT (QTc) duration [22–24].
In contrast, other 5-HT3 RAs have been reported to cause QTc interval prolongation. A comparative study reported that both dolasetron and ondansetron prolonged the QTc interval [19]. Dolasetron was found to predominantly alter ventricular depolarization (QRS interval duration), whereas ondansetron primarily affected ventricular repolarization as measured by a prolongation of QT and JT [25]. Moreover, two studies comparing granisetron and ondansetron in children reported significant prolongation of the QTc interval with granisetron, but not with ondansetron [26, 27].
This study was designed using E14 Guidelines to accurately evaluate the potentially significant implications of cardiovascular toxicity, with the specific aim of detecting the persistent and late electrophysiological variations observed with other 5-HT3 RAs, following the administration of a supratherapeutic dose of palonosetron that is ninefold greater than the current standard dose (2.25 vs 0.25 mg IV). No significant QTc interval prolongations were detected at any time point during the entire study observation period. Supratherapeutic doses of ondansetron and dolasetron both showed a significant dose-dependent effect on the QTc interval for up to 4 h post-administration [25], corresponding to a steady-state condition.
Investigating supratherapeutic dosages may be of particular interest considering the pharmacokinetic profile of palonosetron. Compared with first-generation 5-HT3 RAs, palonosetron has a substantially longer half-life (40 h), which together with its differential receptor binding properties may be associated with its long lasting effects [28–33]. Despite the long exposure to palonosetron, the present thorough QT/QTc E14-compliant trial demonstrated no dose-related cardiotoxicity.
Commonly used chemotherapy regimens, such as those containing anthracyclines, have been associated with cardiac arrhythmias [34]. Since the adverse cardiac effects of some chemotherapeutic agents can emerge even years after treatment, cardiac toxicity of these agents is increasingly relevant because of the rising pool of long-term cancer survivors [34]. The potential for adverse cardiac events should therefore be an important consideration when selecting an antiemetic agent to be administered as supportive care, so that there is no further increase in the potential risk of cardiac complications due to predisposition to cardiac arrhythmias or to cardiotoxic anticancer regimens.
In registrative pivotal trials of palonosetron in both HEC and MEC settings, ECGs were recorded for palonosetron and the comparators dolasetron and ondansetron in order to perform an integrated data analysis. In this analysis, the prolongation effect of palonosetron on QTc was of 2 ms while for ondansetron and dolasetron comparators, the mean changes from baseline were of a larger magnitude of 4–5 ms.
With respect to dolasetron (Anzemet, IV), a dose-dependent increase in QTc interval prolongation was shown in a thorough QT/QTc study [25], resulting in its CINV indication being removed by the US Food and Drug Administration (FDA; 22 September 2011). Post-marketing cases of torsades de pointes were also identified for ondansetron IV at the dose of 32 mg. A thorough QT/QTc study was conducted in which a significant dose relationship with QTc interval prolongation was observed [25]. For this reason, the FDA changed its recommended dose of ondansetron to half of the previously approved dose (a maximum of 16 mg for the IV formulation; 14 November 2014). Published data (ISoP 2011 and 2014) on palonosetron originating from post-marketing spontaneous reporting showed that the number of cardiac events associated with palonosetron was negligible and clinically irrelevant.
Palonosetron caused neither significant clinical prolongation of the QTc interval or adverse cardiac events in this study, indicating, based on ICH E14 guideline principles, that its risk of cardiotoxicity is low and that its use in the prevention of CINV in patients receiving MEC and HEC is apparently safe for cardiac function.
References
de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, Beurden V, Stoter G, Verweij J (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76(8):1055–1061
Small BE, Holdsworth MT, Raisch DW, Winter SS (2000) Survey ranking of emetogenic control in children receiving chemotherapy. J Pediatr Hematol Oncol 22(2):125–132
Grunberg SM (1989) Advances in the management of nausea and vomiting induced by non-cisplatin containing chemotherapeutic regimens. Blood Rev 3(4):216–221
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–v243. doi:10.1093/annonc/mdq194
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH, American Society of Clinical Oncology (2011) Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 29(31):4189–4198
NCCN Clinical Practice Guidelines. Antiemesis. V.1.2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive Accessed 3 Feb 2104
Wilhelm SM, Dehoorne-Smith ML, Kale-Pradham PB (2007) Prevention of postoperative nausea and vomiting. Ann Pharmacother 41(1):68–78
Gussak I, Litwin J, Kleiman R, Grisanti S, Morganroth J (2004) Drug-induced cardiac toxicity: emphasizing the role of electrocardiography in clinical research and drug development. J Electrocardiol 37(1):19–24
Wolbrette DL (2004) Drugs that cause torsades de pointes and increase the risk of sudden cardiac death. Curr Cardiol Rep 6(5):379–384
Cubeddu LX (2003) QT prolongation and fatal arrhythmias: a review of clinical implications and effects of drugs. Am J Ther 10(6):452–457
ICH-E14 guidelines thorough QT study. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf Accessed 3 Feb 2014
Kris MG, Grunberg SM, Gralla RJ, Baltzer L, Zaretsky SA, Lifsey D, Tyson LB, Schmidt L, Hahne WF (1994) Dose-ranging evaluation of the serotonin antagonist dolasetron mesylate in patients receiving high-dose cisplatin. J Clin Oncol 12(5):1045–1049
Turner S, Mathews L, Pandharipande P, Thompson R (2007) Dolasetron-induced torsades de pointes. J Clin Anesth 19(8):622–625
Higgins DJ, Bunker NJ (2005) Dolasetron and peri-operative cardiac arrhythmia. Anaesthesia 60(9):936–937
Rochford M, Kiernan TJ, Aziz A (2007) Dolasetron overdose resulting in prolonged QTc interval and severe hypotension: a case report and literature review. Emerg Med J 24(7):515–517
Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J (2005) Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology 102(6):1094–1100
Afonso N, Dang A, Namshikar V, Kamat S, Rataboli PV (2009) Intravenous ondansetron causing severe bradicardia: two cases. Ann Card Anaesth 12(2):172–173. doi:10.4103/0971-9784.53433
Kasinath N, Malak O, Tetzlaff J (2003) Atrial fibrillation after ondansetron for the prevention and treatment of post-operative nausea and vomiting: a case report. Can J Anaesth 50(3):229–231
Charbit B, Alvarez JC, Dasque E, Abe E, Démolis JL, Funck-Brentano C (2008) Droperidol and ondansetron-induced QT interval prolongation: a clinical drug interaction study. Anesthesiology 109(2):206–212. doi:10.1097/ALN.0b013e31817fd8c8
Avelox®: Summary of Product Characteristics (Swiss Drug Compendium (Schweizer Arzneimittelkompendium), Documed, Basel, Switzerland, 2004)
Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, Tornøe CW, Wang Y, Zhu H, Gobburu JV (2008) Concentration-QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48(1):13–18
Dogan U, Yavas G, Tekinalp M, Yavas C, Ata OY, Ozdemir K (2012) Evaluation of the acute effect of palonosetron on transmural dispersion of myocardial repolarization. Eur Rev Med Pharmacol Sci 16(4):462–468
Gonullu G, Demircan S, Demirag MK, Erdem D, Yucel I (2012) Electrocardiographic findings of palonosetron in cancer patients. Support Care Cancer 20(7):1435–1439. doi:10.1007/s00520-011-1226-5
Yavas C, Dogan U, Yavas G, Araz M, Ata OY (2012) Acute effect of palonosetron on electrocardiographic parameters in cancer patients: a prospective study. Support Care Cancer 20(10):2343–2347. doi:10.1007/s00520-011-1348-9
Benedict CR, Arbogast R, Martin L, Patton L, Morrill B, Hahne W (1996) Single-blind study of the effects of intravenous dolasetron mesylate versus ondansetron on electrocardiographic parameters in normal volunteers. J Cardiovasc Pharmacol 28(1):53–59
Buyukavci M, Olgun H, Ceviz N (2005) The effects of ondansetron and granisetron on electrocardiography in children receiving chemotherapy for acute leukemia. Am J Clin Oncol 28(2):201–204
Pinarli FG, Elli M, Dagdemir A, Baysal K, Acar S (2006) Electrocardiographic findings after 5-HT3 receptor antagonists and chemotherapy in children with cancer. Pediatr Blood Cancer 47(5):567–571
Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J, Rubenstein E, Sebastiani S, Cantoreggi S, Snyder SH, Slusher B (2008) Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg 107(2):469–478. doi:10.1213/ane.0b013e318172fa74
Rojas C, Slusher B (2012) Pharmacological mechanisms of 5-HT3 and tachykinin NK1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol 684(1–3):1–7. doi:10.1016/j.ejphar.2012.01.046
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14(10):1570–1577
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S, 99–04 Palonosetron Study Group (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98(11):2473–2482
Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B, Lordick F, Macciocchi A (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17(9):1441–1449
Saito M, Aogi K, Sekine I et al (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124. doi:10.1016/S1470-2045(08)70313-9
Barry E, Alvarez JA, Scully RE et al (2007) Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother 8(8):1039–1058
Acknowledgments
The authors would like to thank Dr. Gianluca Ballinari for collaboration in writing of the manuscript.
Funding
This work was conducted under the auspices of Helsinn Healthcare SA; 6915, Pambio-Noranco, Lugano Switzerland. Dr. Morganroth served as a consultant to Helsinn. He designed the protocol, interpreted the results, and was integrally involved in the writing of this manuscript. Dr. Flaharty also served as a consultant to Helsinn and had input with regards to pharmacokinetic interpretation and assisted in writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Morganroth, J., Flaharty, K.K., Parisi, S. et al. Effect of single doses of IV palonosetron, up to 2.25 mg, on the QTc interval duration: a double-blind, randomized, parallel group study in healthy volunteers. Support Care Cancer 24, 621–627 (2016). https://doi.org/10.1007/s00520-015-2822-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2822-6