Abstract
Purpose
Disease symptom management in patients with advanced non-small cell lung cancer (NSCLC) is a critical aspect of therapy. The main objective of our study was to assess patient-reported outcomes and the degree of concordance between physician and patient perceptions of symptom severity in advanced NSCLC in the USA.
Methods
Patients with advanced (stage IIIB/IV) NSCLC (N = 450) were recruited in a nationwide (USA) lung cancer study. Patients and their oncologists completed patient and physician versions of the Lung Cancer Symptom Scale (LCSS). Patient-reported lung cancer-specific quality of life was assessed with the Functional Assessment of Cancer Therapy—Lung (FACT-L). Concordance was assessed using the kappa-statistic. Regression analysis was performed with FACT-L total score as the dependent variable and patient-reported LCSS symptom scores as predictors.
Results
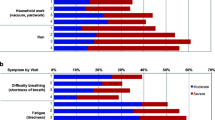
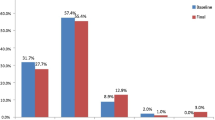
A high proportion of patients experienced lung cancer symptoms: fatigue (100 %), loss of appetite (97 %), shortness of breath (95 %), cough (93 %), pain (92 %), and blood in sputum (63 %). Concordance between physician and patients was lowest for loss of appetite (kappa 0.1701) and greatest for hemoptysis (kappa 0.4586). Loss of appetite (β = −0.204; p < 0.001), cough (β = −0.145; p < 0.01), pain (β = −0.265; p < 0.001), and shortness of breath (β = −0.145; p < 0.01) were found to be significant predictors of the quality of life.
Conclusions
Symptom burden in patients with advanced NSCLC is high and has a negative impact on the quality of life. Patient-reported outcomes data could help optimize disease outcomes and therapy management in NSCLC.


Similar content being viewed by others
References
National Comprehensive Cancer Network (NCCN). Non-small cell lung cancer. NCCN Guidelines, version 3.2012. Available at: http://www.nccn.org. Accessed July 2012
Surveillance Epidemiology and End Results (SEER). SEER Stat Fact Sheets: lung and bronchus. Available at: http://seer.cancer.gov/statfacts/html/lungb.html. Accessed July 2012
Mohan A, Singh P, Singh S, Goyal A, Pathak A, Mohan C, Guleria R (2011) Quality of life in lung cancer patients: impact of baseline clinical profile and respiratory status. Eur J Cancer Care 16:268–276
Xara S, Amaral TF, Parente B (2011) Undernutrition and quality of life in non-small cell lung cancer patients. Rev Port Pneumol 17:153–158
Oi-Ling K, Man-Wah DT, Kam-Hung DN (2005) Symptom distress as rated by advanced cancer patients, caregivers and physicians in the last week of life. Palliat Med 19:228–233
Sikorskii A, Wyatt G, Tamkus D, Victorson D, Rahbar MH, Ahn S (2012) Concordance between patient reports of cancer-related symptoms and medical records documentation. J Pain Symptom Manage 44:362–372
Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, Itri LM, Johnson DH, Scherr SL, Portenoy RK (1997) Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. Semin Hematol 34(3 suppl 2):4–12
Hollen PJ, Gralla RJ, Kris MG, Potanovich LM (1993) Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS). Eur J Cancer 29A(suppl 1):S51–S58
Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P (1995) Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 12:199–220
Landis JR, Koch CG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Quinten C, Maringwa J, Gotay CC, Martinelli F, Coens C, Reeve BB, Flechtner H, Greimel E, King M, Osoba D, Cleeland C, Ringash J, Schmucker-Von Koch J, Taphoorn MJ, Weis J, Bottomley A (2011) Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst 103:1851–1858
Barton JL, Criswell LA, Kaiser R, Chen YH, Schillinger D (2009) Systematic review and metaanalysis of patient self-report versus trained assessor joint counts in rheumatoid arthritis. J Rheumatol 36:2635–2641
Barton JL, Imboden J, Graf J, Glidden D, Yelin EH, Schillinger D (2010) Patient–physician discordance in assessments of global disease severity in rheumatoid arthritis. Arthritis Care Res (Hoboken) 62:857–864
Barbara AM, Loeb M, Dolovich L, Brazil K, Russell M (2012) Agreement between self-report and medical records on signs and symptoms of respiratory illness. Prim Care Respir J 21:145–152
McGrady A, Lynch DJ, Nagel RW, Tamburrino M (2010) Coherence between physician diagnosis and patient self reports of anxiety and depression in primary care. J Nerv Mental Dis 198:420–424
Mohan A, Singh P, Kumar S, Mohan C, Pathak AK, Pandey RM, Guleria R (2008) Effect of change in symptoms, respiratory status, nutritional profile and quality of life on response to treatment for advanced non-small cell lung cancer. Asian Pac J Cancer Prev 9:557–562
Wang XS, Shi Q, Lu C, Basch EM, Johnson VE, Mendoza TR, Mobley GM, Cleeland CS (2010) Prognostic value of symptom burden for overall survival in patients receiving chemotherapy for advanced non-small cell lung cancer. Cancer 116:137–145
Peeters L, Silbille A, Anrys B, Oyen C, Dooms C, Nackaerts K, Wauters I, Vansteenkiste J (2012) Maintenance therapy for advanced non-small cell lung cancer: a pilot study on patients’ perceptions. J Thorac Oncol 7:1291–1295
Bridges JF, Mohamed AF, Finnern HW, Woehl A, Hauber AB (2012) Patients’ preferences for treatment outcomes for advanced non-small cell lung cancer: a conjoint analysis. Lung Cancer 77:224–231
Acknowledgments
The Adelphi Lung Cancer Disease Specific Program was supported by Pfizer and other pharmaceutical companies; the collection of patient-reported outcomes and related analyses was sponsored by Pfizer Inc. Editorial assistance was provided by Martin Quinn at ACUMED® (Tytherington, UK) and funded by Pfizer Inc.
Conflicts of interest
Shrividya Iyer is an employee of Pfizer Inc. Adam Roughley, Alex Rider, and Gavin Taylor-Stokes are employees of Adelphi Real World, who were paid contractors to Pfizer Inc and were involved in study design, data collection, analyses, and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iyer, S., Roughley, A., Rider, A. et al. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer 22, 181–187 (2014). https://doi.org/10.1007/s00520-013-1959-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1959-4