Abstract
Purpose
The aim of this project was to review the literature and define clinical practice guidelines for the use of cytokines and growth factor agents for the prevention or treatment of oral mucositis induced by cancer chemotherapy or radiotherapy.
Methods
A systematic review was conducted by the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO). The body of evidence for each intervention, in each cancer treatment setting, was assigned an evidence level. Based on the evidence level, one of the following three guideline determinations was possible: Recommendation, Suggestion, No guideline possible.
Results
Sixty-four clinical studies across 11 interventions were evaluated. A recommendation was made for the use of recombinant human KGF-1 (palifermin) at a dose of 60 μg/kg per day for 3 days prior to conditioning treatment and for 3 days post-transplant for prevention of oral mucositis in patients receiving high-dose chemotherapy and total body irradiation followed by autologous stem cell transplantation for hematological malignancies. A suggestion was made against using granulocyte macrophage colony-stimulating factor mouthwash for the prevention of oral mucositis in the setting of high-dose chemotherapy followed by autologous or allogeneic stem cell transplantation. No guideline was possible for any other cytokine or growth factor agents due to inconclusive evidence.
Conclusions
Of the cytokine and growth factor agents studied for oral mucositis, the evidence only supports use of palifermin in the specific population listed above. Additional well-designed research is needed on other cytokine and growth factor interventions and in other cancer treatment settings.
Similar content being viewed by others
Introduction
Growth factors and cytokines bind to specific receptors on the cell membrane of target cells. Growth factors are proteins that stimulate cellular growth, proliferation, and differentiation. Cytokines are proteins or glycoproteins that modulate inflammatory and immune responses. There is evidence to suggest that pro-inflammatory cytokines including interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-alpha play an important role in the pathogenesis of mucositis [1].
Growth factors and anti-inflammatory cytokines may be useful in preventing chemotherapy (CT) and/or radiotherapy (RT)-induced mucositis. A number of such agents have been hypothesized to ameliorate the course of mucositis and are described below.
Keratinocyte growth factors (KGF) are members of the fibroblast growth factor (FGF) superfamily. Palifermin is a human recombinant keratinocyte growth factor (KGF-1 or FGF-7) that has pleiotropic activity. It is mitogenic for epithelial and endothelial cells, fibroblasts, and keratinocytes, thereby supporting barrier integrity [2]. Furthermore, KGF-1 is involved in a number of cell survival activities. These include upregulation of the expression of apoptosis regulator B-cell lymphoma-2, which suppresses apoptosis. KGF-1 also activates a redox-sensitive transcription factor, nerve growth factor-2 (Nrf2) that coordinates the expression of cytoprotective genes in cells including keratinocytes, endothelial cells, and fibroblasts. This results in the production of reactive oxygen species-detoxifying enzymes and a modulation of the cellular response to stress [3]. In addition, palifermin upregulates IL-13, an anti-inflammatory cytokine that attenuates the effects of TNF. Although speculative, KGF may downregulate other pro-inflammatory cytokines that are involved in the pathobiology of mucositis [4]. Animal studies suggest that KGF-1 decreases graft-versus-host disease (GVHD) associated with allogeneic hematopoietic stem cell transplantation (HSCT) [5, 6] and enhances T cell reconstitution [7]. Two other members of the KGF family, FGF-20 (velafermin) [8] and human recombinant KGF-2 (repifermin) [9], have overlapping activity with KGF-1 but may also have other actions that impact their effectiveness.
Colony-stimulating factors are specific hematopoietic growth factors needed for bone marrow progenitor cells to form mature blood cells. Granulocyte colony-stimulating factor (G-CSF) stimulates the development of neutrophils, eosinophils, and basophils, whereas granulocyte–macrophage colony-stimulating factor (GM-CSF) stimulates the generation of cells belonging to the monocyte/macrophage lineage. In addition, both G-CSF and GM-CSF enhance the function of peripheral neutrophils, including those in mucosal tissues. GM-CSF has activity on the proliferation of keratinocytes, and animal studies suggest that it enhances wound healing [10]. Direct actions of colony-stimulating factors on peripheral cells as well as a temporal relationship of healing of mucositis and bone marrow recovery have been the rationale for numerous clinical studies testing G-CSF and GM-CSF for the prevention and treatment of oral mucositis.
Epidermal growth factor (EGF) is a polypeptide that plays an important role in maintaining tissue homeostasis as it regulates epithelial cell proliferation, growth, and migration. In addition, EGF enhances mucosal wound healing and tissue generation, suggesting that it may be effective in the treatment of ulcerative oral mucositis [11]. There is evidence to suggest that decreased salivary EGF is associated with more severe RT-induced mucositis [12, 13]. However, EGF and EGF-like peptides are overexpressed in the majority of human carcinomas and are likely involved in the pathogenesis of these tumors. Thus, concerns may be raised on a potential effect of topical EGF on tumor growth, particularly head and neck (H&N) carcinomas.
Transforming growth factor-beta (TGF-β) is part of the transforming growth factor-beta superfamily. TGF-β is a peptide that acts as an antiproliferative factor in many cell types, including epithelial cells and endothelial cells. TGF-β inhibits epithelial cell mitosis by arresting cells in the G1-phase and may thus have the potential to reduce mucositis [14].
Whey-derived growth factor extract contains biologically active proteins including TGF-β, FGF, insulin-like growth factor, and platelet-derived growth factor [15].
IL-11 is a pleiotropic cytokine that can be isolated from bone marrow-derived stromal cells. It is a key regulator of multiple events in hematopoiesis, most notably the stimulation of megakaryocyte maturation. In murine HSCT models, IL-11 reduces gut permeability, induces T helper-2 cell differentiation, and accelerates recovery of oral and bowel mucosa [16]. IL-11 also favorably modulated RT-induced oral mucositis in a hamster model by attenuating pro-inflammatory cytokine expression [17].
ATL-104 is a potent plant lectin mitogen for epithelial cells of the gastrointestinal tract. In an animal model, ATL-104 aids regeneration of CT-induced damage to the gastrointestinal tract [18].
The trefoil factor (TFF) family comprises a group of small growth factor-like peptides, which are highly expressed in tissues containing mucus-producing cells, particularly the mucosa lining the gastrointestinal tract. Although not a growth factor per se, TFF plays a role in maintaining mucosal integrity and repairing damaged mucosa and was therefore included in this review. In vitro studies have shown that TFF peptides prevent tissue damage by multiple mechanisms, including forming a gel-like matrix by cross-linking with mucins. Therapeutic effects of TFF have been shown in several animal models of gastrointestinal damage [19].
The Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) has published clinical practice guidelines for mucositis [20], [21]. These have resulted in a recommendation for the use of palifermin at a dose of 60 μg/kg per day for 3 days prior to conditioning treatment and for 3 days post-transplant to prevent oral mucositis in patients receiving high-dose CT and total body irradiation (TBI) followed by autologous stem cell transplantation for hematological malignancies. A suggestion has also been made against using GM-CSF mouthwash for the prevention of oral mucositis following CT in the transplant setting, since this agent was not found to be effective. No other guidelines for this class of agents have been possible to date due to insufficient or conflicting data.
As part of a comprehensive update of the MASCC/ISOO clinical practice guidelines for mucositis, the aim of this project was to systematically review the available literature and define evidence-based clinical practice guidelines for the use of cytokine and growth factor agents for the prevention and treatment of mucositis.
Methods
The methods are described in detail in papers by Bowen et al. [22] and Elad et al. [23] published elsewhere in this issue. Briefly, a literature search for relevant papers published before 31st December 2010 was conducted using OVID/MEDLINE, with papers selected for review based on defined inclusion and exclusion criteria.
Papers were reviewed by two independent reviewers, and data was extracted using a standard electronic form. Studies were scored for their Level of Evidence based on Somerfield criteria [24], and flaws were listed according to Hadorn criteria [25]. A well-designed study was defined as a study with no major flaws per Hadorn criteria.
Following panel consensus, findings from the reviewed studies were integrated into guidelines based on the overall Level of Evidence for each intervention. Guidelines were classified into three types: recommendation, suggestion, and no guideline possible. Guidelines were separated based on (1) the aim of the intervention (prevention or treatment of mucositis); (2) the treatment modality (RT, CT, chemoradiation, or high-dose conditioning therapy for HSCT); and (3) the route of administration of the intervention.
The list of intervention keywords used for the literature search of this section included: growth substances, cytokines, immunologic factors, colony-stimulating factors, amino acids, fibroblast growth factors, transforming growth factors, epidermal growth factor, platelet-derived growth factor, hepatocyte growth factor, vascular endothelial growth factor, somatomedins, interleukins, erythropoietin, granulocyte colony-stimulating factor, granulocyte–macrophage colony-stimulating factor, macrophage colony-stimulating factor, thrombopoietin, ghrelin, keratinocyte growth factor, palifermin, milk-derived protein, whey protein, milk-derived growth factor extract, PV701, glucagon-like peptide 2, teduglutide, intestinal trefoil factor, carcinoembryonic antigen cell adhesion molecule 1, glutathione, FGF-7, FGF-20, CG 53135, velafermin, repifermin, and insuline-like growth factor.
In addition, the references of review papers were searched. We included papers reporting clinical studies with interventions including: palifermin, velafermin, repifermin, G-CSF, GM-CSF, EGF, TGF-beta, milk-derived growth factor extract, IL-11, ATL-10, and recombinant intestinal trefoil factor. We compared our findings with those of three systematic review papers including meta-analyses on mucositis interventions.
Results
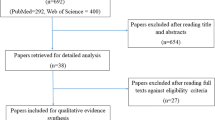
Database searches found 1,718 papers. The full text of 156 papers was retrieved for detailed analysis, of which 31 were excluded immediately for not matching the inclusion criteria. Of the 125 remaining articles, the full text was assessed for methodological quality; 61 papers were removed based on failure to meet inclusion criteria, and 64 clinical studies were included in the review. Furthermore, three systematic reviews including meta-analyses on cytokines and growth factors were identified. Three studies were published after the cut-off date and are discussed as late breaking reports.
Agents belonging to the fibroblast growth factors superfamily
As summarized in Table 1, we continue recommending KGF-1 (palifermin) for the prevention of oral mucositis in patients with hematological malignancies receiving high-dose CT and TBI followed by autologous HSCT. Palifermin is administered intravenously in a dose of 60 μg/kg per day for 3 days prior to conditioning treatment and for 3 days post-transplantation. Our recommendation is based on the findings of a well-designed randomized clinical trial (RCT) [26, 27]. Evidence on the efficacy of palifermin in autologous HSCT without TBI conditioning is conflicting [28–34], and these rather small studies did not allow a guideline. In addition, no guideline could be provided for the use of palifermin in the setting of allogeneic HSCT with or without TBI [28, 35–37]. No guideline could be provided for the use of palifermin in the setting of CT for solid and hematological tumors [38–41] due to insufficient evidence, although a single center RCT of 49 patients, using a single dose of palifermin (180 μg/kg) before each cycle of CT prevented mucositis in multicycle CT for sarcoma [41]. In addition, no guideline could be provided for the use of palifermin in H&N RT due to insufficient evidence [42].
The meta-analysis performed by Worthington, and coworkers found a statistically significant benefit for palifermin to reduce the incidence of oral mucositis [43]. The meta-analysis included all available studies but did not discriminate between different clinical settings.
Studies performed on FGF-20 (velafermin) [8] and KGF-2 (repifermin) [9] did not allow a guideline due to insufficient evidence. The study on velafermin was a single center, phase I, open label, dose escalation study assessing the safety and tolerability of this growth factor. Similarly, the primary endpoint of the study on repifermin was to evaluate its safety. A preliminary analysis of data and patient-reported outcomes indicated that repifermin was well tolerated and seemed active in reducing oral mucositis. Nevertheless, both velafermin and repifermin did not become available on the market.
Granulocyte colony-stimulating factor
In our previous update, we were not able to provide a guideline for the use of subcutaneous G-CSF for the prevention of oral mucositis in patients treated with chemoradiation for H&N cancers. Two cohort studies did not find a benefit for the use of this growth factor [44, 45] in these patients, whereas a small study by Schneider et al. [46] reported only preliminary results. Since then, only one study on the use of systemic G-CSF for the prevention of oral mucositis induced by (C)RT for H&N cancers has been published [47]. This study reported a non-significant trend for a beneficial effect of this intervention but was closed prematurely because of low accrual. We did not change our previous conclusion that no guideline was possible because of insufficient evidence. The panel concluded that no guideline could be provided for or against the use of subcutaneous G-CSF for the prevention of mucositis in patients treated with CT since studies reported conflicting results [48, 49]. Crawford et al. [50] reported a beneficial effect, whereas a randomized controlled trial by Patte et al. [51] found that G-CSF was not effective to prevent mucositis in this setting (Table 1). In addition, no guideline could be provided for the use of a G-CSF mouthwash for the prevention of CT-induced oral mucositis [52].
The meta-analysis performed by Stokman et al. [53] concluded that systemic G(M)-CSF may prevent oral mucositis. Worthington et al. [43] concluded that there is weak evidence that systemic or topical G-CSF may be beneficial for the prevention of severe OM in H&N cancer patients undergoing RT.
Granulocyte–macrophage colony-stimulating factor
Our previous systematic review provided a suggestion against using GM-CSF mouthwash for the prevention of oral mucositis in patients undergoing autologous or allogeneic HSCT [21]. This conclusion was mainly based on the results of a robust RCT by Dazzi et al. [54]. In the present update, an additional RCT by van der Lelie et al. [55] has been included, which provided additional evidence that GM-CSF mouthwashes are not effective to prevent oral mucositis in the HSCT setting. We continue to suggest not using preventative GM-CSF mouthwashes in these patients (Table 1). A well-designed dosing study for preventative GM-CSF mouthwashes by Cartee et al. [56] found no clear benefit for the use of this agent in patients treated with CT (including 5-fluorouracil, adriamycin, and methotrexate) for metastatic breast cancer. However, the panel decided that a guideline against the use of GM-CSF mouthrinses in all patients treated with various stomatotoxic CT regimens was not possible, since only one site-specific and highly mucotoxic CT regimen was evaluated in this study. Nicolatou-Galitis et al. [57, 58] reported results from a case series suggesting a preventative effect of GM-CSF mouthwashes on mucositis in patients receiving RT for H&N cancer, whereas controlled studies by Saarilahti et al. and Mantovani et al. [59, 60] reported only a marginal effect. In contrast, Sprinzl et al. [61] found no benefit (Table 1). Because of these conflicting results, no guideline could be provided for the use of GM-CSF mouthwashes for the prevention of (C)RT-induced oral mucositis. Prevention of mucositis using systemically administered GM-CSF has also been tested. One study found a benefit of this drug in H&N cancer patients treated with CT [62]. Whereas some studies found a benefit for preventative use of systemic GM-CSF in H&N cancer patients undergoing (C)RT [63–66], others did not confirm such effect [67, 68]. In addition, two studies indicated a benefit for systemically administered GM-CSF to prevent mucositis in the adult HSCT setting [69, 70], although in the latter study, oral mucositis was not a primary outcome. Gordon et al. [71] reported decreased duration of oral mucositis in pediatric HSCT with systemic GM-CSF. In sum, the panel concluded that the available evidence did not allow a guideline for the use of systemic GM-CSF to prevent oral mucositis associated with any of these cancer treatments.
Several studies addressed the use of GM-CSF mouthwashes for the treatment of established mucostitis in patients receiving (C)RT for H&N tumors [60, 72], HSCT[73, 74], and CT [75, 76]. Due to insufficient and conflicting evidence, no guideline was possible for the use of GM-CSF mouthwashes for the treatment of oral mucositis in any of these settings. In addition, there was not enough evidence to provide a guideline for the use of systemic GM-CSF for the treatment of oral mucositis in patients receiving H&N RT [77] or CT [78].
Clarkson et al. [79] and Worthington et al. [43] concluded that topical or systemic GM-CSF cannot be recommended for prevention or treatment of oral mucositis.
Transforming growth factor-beta, milk-derived growth factor extract, epidermal growth factor, interleukin-11, ATL-104 mitogen, and recombinant human intestinal trefoil factor
As shown in Table 1, no guidelines could be provided for any of these interventions due to insufficient evidence. TGF-β mouthwashes or enriched food were not effective to reduce oral mucositis in the used formulations [80–82]. A mouthwash with bovine milk-derived whey growth factors (PV-701) was well tolerated, and as compared to historical controls, the severity as well as the duration of oral mucositis seemed to be decreased in autologous HSCT recipients [83].
With respect to the use of topical EGF, the panel expressed concerns about the potential unfavorable effects of this growth factor on tumor growth [84–86], whereas the use of subcutaneous IL-11 was associated with severe side effects and mortality [87]. ATL-104 mouthwash reduced the duration of oral mucositis, whereas its effect on the incidence of mucositis was unclear [88]. Recombinant human intestinal trefoil factor oral spray was found to be safe and effective for the reduction of CT-induced oral mucositis in patients with colorectal cancers [89], but these limited data did not permit a guideline.
Discussion and late breaking reports
Although new evidence was included in the review process of the present update, this did not result in any changes of the clinical practice guidelines for cytokines and growth factor agents for the management of mucositis since the 2005 MASCC/ISOO review process [20, 21].
A suggestion was provided for not using GM-CSF mouthwash for the prevention of oral mucositis in patients undergoing autologous or allogeneic HSCT. With respect to the use of GM-CSF for the prevention or treatment of oral mucositis in other patient populations, we were not able to provide guidelines because of insufficient evidence (i.e., major flaws in study design and/or methods, according to the Hadorn criteria [25] and/or conflicting results).
We continue recommending the use of palifermin at a dose of 60 μg/kg per day for 3 days prior to conditioning treatment and for 3 days post-transplant to prevent oral mucositis in patients receiving high-dose chemotherapy and TBI followed by autologous stem cell transplantation for hematological malignancies, based on a well-designed RCT [26]. Although palifermin has been shown to be efficacious in this specific high-toxicity regimen, optimal dosing and timing of palifermin administration may be crucial and is likely to be different among different conditioning regimens.
In a high-dose melphalan conditioning regimen without TBI followed by autologous HSCT, the interval between the doses was shorter than in the registration study, and palifermin was associated with unfavorable side effects, including skin problems, orofacial swelling, mucosal ulceration, and taste alterations [33]. These observations warrant further investigation. The question whether palifermin reduces GVHD has been addressed in a number of clinical trials of allogeneic HSCT following myeloablative conditioning using cyclophosphamide plus TBI or CT only. None showed an impact on the occurrence of acute GVHD [28, 35, 37] or chronic GVHD [90]. The impact on mucositis was not consistent, with one study showing a decrease of oral mucositis only in patients conditioned with cyclophosphamide and TBI, but not in patients receiving a less mucotoxic regimen of busulfan and cyclophosphamide [35]. These findings are in line with a retrospective study on 251 patients published after the cut-off date for this review [91]. No mucositis measurements were available, but following palifermin administration similar to the registration study [26], mucositis-related adverse outcomes, including the mean number of days of total parenteral nutrition (13 versus 17 days, P < 0.001), duration of patient-controlled analgesia (7 versus 12 days, P = 0.033) and length of hospital stay (32 versus 38 days, P = 0.001) were significantly decreased in TBI-based, but not in CT-based allogeneic HSCT. Palifermin did not affect GVHD.
No guideline could be provided for the use of palifermin in the setting of CT for solid and hematological tumors due to insufficient evidence [38–41], although the results of the single center study by Vadham-Raj et al. [41] are promising to prevent mucositis in multicycle CT for sarcoma.
In addition to the results of the study by Brizel et al. [42], in which post hoc analysis suggested that palifermin was marginally effective to reduce the duration of oral mucositis in hyperfractionated RT for H&N tumors, two publications of large multicenter, double-blind RCTs in patients treated with conventional 3D-chemoradiotherapy for H&N cancers became available after the cut-off date for the present update [92, 93]. Henke et al. [92] reported on patients that underwent postoperative CRT and received palifermin at doses of 120 μg/kg/week from 3 days before and continuing throughout the duration of treatment. A significant reduction of severe mucositis (WHO grades 3 and 4) was found in the treatment group (51 % versus 67 % in controls; P = 0.027). Palifermin also delayed onset and significantly decreased the duration of severe mucositis (median 4.5 versus 22 days). However, patient-reported outcomes and treatment breaks did not differ between the treatment arms. Le et al. performed a parallel study of palifermin administered to patients undergoing definitive chemoradiotherapy for locally advanced H&N cancer using 180 μg/kg of palifermin or placebo prior to therapy and then weekly for 7 weeks [93]. In this study, the incidence of severe mucositis was also reduced in the treatment group (54 % versus 69 % in controls; P = 0.041), the time to develop severe mucositis was longer, and the duration of severe mucositis was decreased in the palifermin arm. Disappointingly, other clinically relevant outcomes including mouth and throat soreness, opioid use, and treatment breaks did not differ significantly between the two arms. These studies suggest that palifermin may decrease the incidence of mucositis in H&N cancer patients treated with chemoradiotherapy based upon WHO scoring, while the impact upon patient symptoms is not clear. Palifermin did not affect survival observed 42 months post treatment, but follow-up studies are needed to confirm that the use of palifermin does not negatively affect tumor control and survival rates.
Recommendations for future research
Additional well-designed research is needed on cytokine and growth factor interventions. The clinical success of these biological agents depends on the choice of formulation, timing, route of administration, dosing, and stability of these agents. Furthermore, it is important to gain more insight into the pathobiology of mucositis as well as into pharmacogenetic variables and other genetic differences that underpin mucositis susceptibility.
TFF peptides protect mucosal cells from injury as well as contribute to epithelial reconstitution and should be considered as promising anti-mucositis agents [89]. A preclinical study suggested that a mouth rinse with genetically modified bacteria engineered to secrete human TFF-1 may provide future management tools [94].
Additional studies are necessary to assess the role of palifermin in patients treated for H&N cancers, particularly as therapeutic approaches continue to evolve. An intriguing development is the concept of hKGF gene transfer to salivary glands. In murine models, transgenic hKGF secreted from vector-transduced submandibular glands effectively protected oral mucosal epithelial cells from radiation injury [95]. This route of administration may prove to be beneficial in the future to prevent oral mucositis in patients being treated for H&N cancers.
Future studies should also include evaluating the safety and efficacy of using cytokines and growth factor agents for the management of mucositis in children and adolescents. In addition, future studies on these agents should not only focus on oral mucositis but should also be directed to protection from esophageal and gut mucosal barrier injury. However, cytokine and growth factor agents may have undesirable effects that may result from either stimulation of tumor growth or by interference with the tumor response to treatment. Therefore, the absence of tumor growth following the administration of stimulatory cytokines and growth factors must be confirmed in long-term follow-up studies.
References
Logan RM, Stringer AM, Bowen JM, Yeoh AS, Gibson RJ, Sonis ST, Keefe DM (2007) The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 33(5):448–460
Farrell CL, Rex KL, Chen JN, Bready JV, DiPalma CR, Kaufman SA, Rattan A, Scully S, Lacey DL (2002) The effects of keratinocyte growth factor in preclinical models of mucositis. Cell Prolif 35(Suppl 1):78–85
Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100(9 Suppl):1995–2025
Sonis ST (2004) The pathobiology of mucositis. Nat Rev Cancer 4(4):277–284
Krijanovski OI, Hill GR, Cooke KR, Teshima T, Crawford JM, Brinson YS, Ferrara JL (1999) Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood 94(2):825–831
Panoskaltsis-Mortari A, Lacey DL, Vallera DA, Blazar BR (1998) Keratinocyte growth factor administered before conditioning ameliorates graft-versus-host disease after allogeneic bone marrow transplantation in mice. Blood 92(10):3960–3967
Seggewiss R, Lore K, Guenaga FJ, Pittaluga S, Mattapallil J, Chow CK, Koup RA, Camphausen K, Nason MC, Meier-Schellersheim M, Donahue RE, Blazar BR, Dunbar CE, Douek DC (2007) Keratinocyte growth factor augments immune reconstitution after autologous hematopoietic progenitor cell transplantation in rhesus macaques. Blood 110(1):441–449
Schuster MW, Shore TB, Harpel JG, Greenberg J, Jalilizeinali B, Possley S, Gerwien RW, Hahne W, Halvorsen YD (2008) Safety and tolerability of velafermin (CG53135-05) in patients receiving high-dose chemotherapy and autologous peripheral blood stem cell transplant. Support Care Cancer 16(5):477–483
Freytes CO, Ratanatharathorn V, Taylor C, Abboud C, Chesser N, Restrepo A, Arango J, Odenheimer D (2004) Phase I/II randomized trial evaluating the safety and clinical effects of repifermin administered to reduce mucositis in patients undergoing autologous hematopoietic stem cell transplantation. Clin Cancer Res 10(24):8318–8324
Jyung RW, Wu L, Pierce GF, Mustoe TA (1994) Granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor: differential action on incisional wound healing. Surgery 115(3):325–334
Noguchi S, Ohba Y, Oka T (1991) Effect of salivary epidermal growth factor on wound healing of tongue in mice. Am J Physiol 260(4):E620–E625
Epstein JB, Emerton S, Guglietta A, Le N (1997) Assessment of epidermal growth factor in oral secretions of patients receiving radiation therapy for cancer. Oral Oncol 33(5):359–363
Epstein JB, Gorsky M, Guglietta A, Le N, Sonis ST (2000) The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer 89(11):2258–2265
Sonis ST, Van Vugt AG, Brien JP, Muska AD, Bruskin AM, Rose A, Haley JD (1997) Transforming growth factor-beta 3 mediated modulation of cell cycling and attenuation of 5-fluorouracil induced oral mucositis. Oral Oncol 33(1):47–54
Taylor VL, Goddard C, Read LC (2001) A milk growth factor extract reduces chemotherapeutic drug toxicity in epithelial cells in vitro. In Vitro Cell Dev Biol Anim 37(5):310–318
Orazi A, Du X, Yang Z, Kashai M, Williams DA (1996) Interleukin-11 prevents apoptosis and accelerates recovery of small intestinal mucosa in mice treated with combined chemotherapy and radiation. Lab Invest 75(1):33–42
Sonis ST, Peterson RL, Edwards LJ, Lucey CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P, Hayes LL, Bedrosian C, Dorner AJ (2000) Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol 36(4):373–381
Linderoth A, Biernat M, Prykhodko O, Kornilovska I, Pusztai A, Pierzynowski SG, Bjorn WR (2005) Induced growth and maturation of the gastrointestinal tract after Phaseolus vulgaris lectin exposure in suckling rats. J Pediatr Gastroenterol Nutr 41(2):195–203
Kjellev S (2009) The trefoil factor family—small peptides with multiple functionalities. Cell Mol Life Sci 66(8):1350–1369
von Bultzingslowen I, Brennan MT, Spijkervet FK, Logan R, Stringer A, Raber-Durlacher JE, Keefe D (2006) Growth factors and cytokines in the prevention and treatment of oral and gastrointestinal mucositis. Support Care Cancer 14(6):519–527
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109(5):820–831
Bowen JM, Elad S, Hutchins RD, Lalla RV (2012) Methodology for the MASCC/ISOO Mucositis Clinical Practice Guidelines Update. Supportive Care in Cancer (in press)
Elad S, Bowen JM, Zadik Y, Lalla RV (2012) Development of the MASCC/ISOO Mucositis Guidelines: Considerations Underlying the Process. Supportive Care in Cancer (in press)
Somerfield MR, Einhaus K, Hagerty KL, Brouwers MC, Seidenfeld J, Lyman GH (2008) American Society of Clinical Oncology clinical practice guidelines: opportunities and challenges. Journal of Clinical Oncology: Official Journal of the Am Soc Clin Oncol 26(24):4022–4026
Hadorn DC, Baker D, Hodges JS, Hicks N (1996) Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol 49(7):749–754
Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, Shea T, Yanovich S, Hansen K, Noga S, McCarty J, LeMaistre CF, Sung EC, Blazar BR, Elhardt D, Chen MG, Emmanouilides C (2004) Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 351(25):2590–2598
Stiff PJ, Emmanouilides C, Bensinger WI, Gentile T, Blazar B, Shea TC, Lu J, Isitt J, Cesano A, Spielberger R (2006) Palifermin reduces patient-reported mouth and throat soreness and improves patient functioning in the hematopoietic stem-cell transplantation setting. Journal of Clinical Oncology: Official Journal of the Am Soc Clin Oncol 24(33):5186–5193
Nasilowska-Adamska B, Rzepecki P, Manko J, Czyz A, Markiewicz M, Federowicz I, Tomaszewska A, Piatkowska-Jakubas B, Wrzesien-Kus A, Bieniaszewska M, Duda D, Szydlo R, Halaburda K, Szczepinski A, Lange A, Hellman A, Robak T, Skotnicki A, Jedrzejczak WW, Walewski J, Holowiecki J, Komarnicki M, Dmoszynska A, Warzocha K, Marianska B (2007) The influence of palifermin (Kepivance) on oral mucositis and acute graft versus host disease in patients with hematological diseases undergoing hematopoietic stem cell transplant. Bone Marrow Transplant 40(10):983–988
Keefe D, Lees J, Horvath N (2006) Palifermin for oral mucositis in the high-dose chemotherapy and stem cell transplant setting: the Royal Adelaide Hospital Cancer Centre experience. Support Care Cancer 14(6):580–582
Tsirigotis P, Triantafyllou K, Girkas K, Giannopoulou V, Ioannidou E, Chondropoulos S, Kalli T, Papaxoinis G, Pappa V, Papageorgiou E, Economopoulos T, Ladas SD, Dervenoulas J (2008) Keratinocyte growth factor is effective in the prevention of intestinal mucositis in patients with hematological malignancies treated with high-dose chemotherapy and autologous hematopoietic SCT: a video-capsule endoscopy study. Bone Marrow Transplant 42(5):337–343
Horsley P, Bauer JD, Mazkowiack R, Gardner R, Bashford J (2007) Palifermin improves severe mucositis, swallowing problems, nutrition impact symptoms, and length of stay in patients undergoing hematopoietic stem cell transplantation. Support Care Cancer 15(1):105–109
Johansson JE, Hasseus B, Johansson P, Eklof C, Ohman D, Stockelberg D (2009) Gut protection by palifermin during autologous haematopoietic SCT. Bone Marrow Transplant 43(10):807–811
Verhagen MP, Wondergem MJ, Visser O (2009) Palifermin dose should be adjusted to different therapy regimens. Bone Marrow Transplant 43(8):665
Kobbe G, Bruns I, Schroeder T, Czibere A, Warnecke J, Hieronimus N, Safaian N, Kondakci M, Saure C, Germing U, Haas R, Fenk R (2010) A 3-day short course of palifermin before HDT reduces toxicity and need for supportive care after autologous blood stem-cell transplantation in patients with multiple myeloma. Ann Oncol 21(9):1898–1904
Blazar BR, Weisdorf DJ, Defor T, Goldman A, Braun T, Silver S, Ferrara JL (2006) Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood 108(9):3216–3222
Rzepecki P, Sarosiek T, Barzal J, Oborska S, Nurzynski P, Wasko A, Szczylik C (2007) Palifermin for prevention of oral mucositis after haematopoietic stem cell transplantation—single centre experience. J BUON 12(4):477–482
Langner S, Staber P, Schub N, Gramatzki M, Grothe W, Behre G, Rabitsch W, Urban C, Linkesch W, Neumeister P (2008) Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients. Bone Marrow Transplant 42(4):275–279
Meropol NJ, Somer RA, Gutheil J, Pelley RJ, Modiano MR, Rowinsky EK, Rothenberg ML, Redding SW, Serdar CM, Yao B, Heard R, Rosen LS (2003) Randomized phase I trial of recombinant human keratinocyte growth factor plus chemotherapy: potential role as mucosal protectant. Journal of Clinical Oncology: Official Journal of the Am Soc Clin Oncol 21(8):1452–1458
Rosen LS, Abdi E, Davis ID, Gutheil J, Schnell FM, Zalcberg J, Cesano A, Gayko U, Chen MG, Clarke S (2006) Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. Journal of Clinical Oncology: Official Journal of the Am Soc Clin Oncol 24(33):5194–5200
Schmidt E, Thoennissen NH, Rudat A, Bieker R, Schliemann C, Mesters RM, Zuhlsdorf M, Muller-Tidow C, Berdel WE (2008) Use of palifermin for the prevention of high-dose methotrexate-induced oral mucositis. Ann Oncol 19(9):1644–1649
Vadhan-Raj S, Trent J, Patel S, Zhou X, Johnson MM, Araujo D, Ludwig JA, O'Roark S, Gillenwater AM, Bueso-Ramos C, El-Naggar AK, Benjamin RS (2010) Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomized trial. Ann Intern Med 153(6):358–367
Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Gluck S, Brizel HE, Meredith RF, Berger D, Chen MG, Mendenhall W (2008) Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. Journal of Clinical Oncology: Official Journal of the Am Soc Clin Oncol 26(15):2489–2496
Worthington HV, Clarkson JE, Bryan G, Furness S, Glenny AM, Littlewood A, McCabe MG, Meyer S, Khalid T (2010) Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev (12):CD000978
Abitbol AA, Sridhar KS, Lewin AA, Schwade JG, Raub W Jr, Wolfson A, Gonzalez-Angulo C, Adessa A, Goodwin WJ, Markoe AM (1997) Hyperfractionated radiation therapy and 5-fluorouracil, cisplatin, and mitomycin-C (+/− granulocyte-colony stimulating factor) in the treatment of patients with locally advanced head and neck carcinoma. Cancer 80(2):266–276
Mascarin M, Franchin G, Minatel E, Gobitti C, Talamini R, De Maria D, Trovo MG (1999) The effect of granulocyte colony-stimulating factor on oral mucositis in head and neck cancer patients treated with hyperfractionated radiotherapy. Oral Oncol 35(2):203–208
Schneider SB, Nishimura RD, Zimmerman RP, Tran L, Shiplacoff J, Tormey M, Contreras R, Juillard GF (1999) Filgrastim (r-metHuG-CSF) and its potential use in the reduction of radiation-induced oropharyngeal mucositis: an interim look at a randomized, double-blind, placebo-controlled trial. Cytokines Cell Mol Ther 5(3):175–180
Su YB, Vickers AJ, Zelefsky MJ, Kraus DH, Shaha AR, Shah JP, Serio AM, Harrison LB, Bosl GJ, Pfister DG (2006) Double-blind, placebo-controlled, randomized trial of granulocyte-colony stimulating factor during postoperative radiotherapy for squamous head and neck cancer. Cancer J 12(3):182–188
Katano M, Nakamura M, Matsuo T, Iyama A, Hisatsugu T (1995) Effect of granulocyte colony-stimulating factor (G-CSF) on chemotherapy-induced oral mucositis. Surg Today 25(3):202–206
Viens P, Gravis G, Bladou F, Lechevallier E, Baume D, Camerlo J, Cowen D, Coulange C, Serment G, Resbeut M, Maraninchi D (1996) Impact of recombinant human granulocyte colony stimulating factor on dose intensity and toxicity of three cycles of methotrexate, vinblastine, doxorubicin and cisplatin in patients with previously untreated urothelial bladder carcinoma. Eur Cytokine Netw 7(3):395–399
Crawford J, Tomita DK, Mazanet R, Glaspy J, Ozer H (1999) Reduction of oral mucositis by filgrastim (r-metHuG-CSF) in patients receiving chemotherapy. Cytokines Cell Mol Ther 5(4):187–193
Patte C, Laplanche A, Bertozzi AI, Baruchel A, Frappaz D, Schmitt C, Mechinaud F, Nelken B, Boutard P, Michon J (2002) Granulocyte colony-stimulating factor in induction treatment of children with non-Hodgkin's lymphoma: a randomized study of the French Society of Pediatric Oncology. Journal of Clinical Oncology: Official Journal of the Am Soc Clin Oncol 20(2):441–448
Karthaus M, Rosenthal C, Huebner G, Paul H, Elser C, Hertenstein B, Krauter J, Scharmann T, Geissler RG, Heil G, Ganser A (1998) Effect of topical oral G-CSF on oral mucositis: a randomised placebo-controlled trial. Bone Marrow Transplant 22(8):781–785
Stokman MA, Spijkervet FK, Boezen HM, Schouten JP, Roodenburg JL, de Vries EG (2006) Preventive intervention possibilities in radiotherapy- and chemotherapy-induced oral mucositis: results of meta-analyses. J Dent Res 85(8):690–700
Dazzi C, Cariello A, Giovanis P, Monti M, Vertogen B, Leoni M, Tienghi A, Turci D, Rosti G, Nanni O, Rondoni C, Marangolo M (2003) Prophylaxis with GM-CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high-dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: a double blind, randomized, placebo-controlled study. Ann Oncol 14(4):559–563
van der Lelie H, Thomas BL, van Oers RH, Ek-Post M, Sjamsoedin SA, van Dijk-Overtoom ML, Timmer JG, von dem Borne AE (2001) Effect of locally applied GM-CSF on oral mucositis after stem cell transplantation: a prospective placebo-controlled double-blind study. Ann Hematol 80(3):150–154
Cartee L, Petros WP, Rosner GL, Gilbert C, Moore S, Affronti ML, Hoke JA, Hussein AM, Ross M, Rubin P et al (1995) Evaluation of GM-CSF mouthwash for prevention of chemotherapy-induced mucositis: a randomized, double-blind, dose-ranging study. Cytokine 7(5):471–477
Nicolatou O, Sotiropoulou-Lontou A, Skarlatos J, Kyprianou K, Kolitsi G, Dardoufas K (1998) A pilot study of the effect of granulocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients during X-radiation therapy: a preliminary report. Int J Radiat Oncol Biol Phys 42(3):551–556
Nicolatou-Galitis O, Dardoufas K, Markoulatos P, Sotiropoulou-Lontou A, Kyprianou K, Kolitsi G, Pissakas G, Skarleas C, Kouloulias V, Papanicolaou V, Legakis NJ, Velegraki A (2001) Oral pseudomembranous candidiasis, herpes simplex virus-1 infection, and oral mucositis in head and neck cancer patients receiving radiotherapy and granulocyte-macrophage colony-stimulating factor (GM-CSF) mouthwash. J Oral Pathol Med 8:471–480
Saarilahti K, Kajanti M, Joensuu T, Kouri M, Joensuu H (2002) Comparison of granulocyte-macrophage colony-stimulating factor and sucralfate mouthwashes in the prevention of radiation-induced mucositis: a double-blind prospective randomized phase III study. Int J Radiat Oncol Biol Phys 54(2):479–485
Mantovani G, Massa E, Astara G, Murgia V, Gramignano G, Lusso MR, Camboni P, Ferreli L, Mocci M, Perboni S, Mura L, Madeddu C, Maccio A (2003) Phase II clinical trial of local use of GM-CSF for prevention and treatment of chemotherapy- and concomitant chemoradiotherapy-induced severe oral mucositis in advanced head and neck cancer patients: an evaluation of effectiveness, safety and costs. Oncol Rep 10(1):197–206
Sprinzl GM, Galvan O, de Vries A, Ulmer H, Gunkel AR, Lukas P, Thumfart WF (2001) Local application of granulocyte-macrophage colony stimulating factor (GM-CSF) for the treatment of oral mucositis. Eur J Cancer 37(16):2003–2009
Chi KH, Chen CH, Chan WK, Chow KC, Chen SY, Yen SH, Chao JY, Chang CY, Chen KY (1995) Effect of granulocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients after cisplatin, fluorouracil, and leucovorin chemotherapy. Journal of clinical oncology: official journal of the Am Soc Clin Oncol 13(10):2620–2628
Kannan V, Bapsy PP, Anantha N, Doval DC, Vaithianathan H, Banumathy G, Reddy KB, Kumaraswamy SV, Shenoy AM (1997) Efficacy and safety of granulocyte macrophage-colony stimulating factor (GM-CSF) on the frequency and severity of radiation mucositis in patients with head and neck carcinoma. Int J Radiat Oncol Biol Phys 37(5):1005–1010
Rosso M, Blasi G, Gherlone E, Rosso R (1997) Effect of granulocyte-macrophage colony-stimulating factor on prevention of mucositis in head and neck cancer patients treated with chemo-radiotherapy. J Chemother 9(5):382–385
Wagner W, Alfrink M, Haus U, Matt J (1999) Treatment of irradiation-induced mucositis with growth factors (rhGM-CSF) in patients with head and neck cancer. Anticancer Res 19(1B):799–803
McAleese JJ, Bishop KM, A'Hern R, Henk JM (2006) Randomized phase II study of GM-CSF to reduce mucositis caused by accelerated radiotherapy of laryngeal cancer. Br J Radiol 79(943):608–613
Ryu JK, Swann S, LeVeque F, Scarantino CW, Johnson D, Chen A, Fortin A, Pollock J, Kim H, Ang KK (2007) The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: a double-blind placebo-controlled prospective phase III study by Radiation Therapy Oncology Group 9901. Int J Radiat Oncol Biol Phys 67(3):643–650
Makkonen TA, Minn H, Jekunen A, Vilja P, Tuominen J, Joensuu H (2000) Granulocyte macrophage-colony stimulating factor (GM-CSF) and sucralfate in prevention of radiation-induced mucositis: a prospective randomized study. Int J Radiat Oncol Biol Phys 46(3):525–534
Ifrah N, Witz F, Jouet JP, Francois S, Lamy T, Linassier C, Pignon B, Berthou C, Guyotat D, Cahn JY, Harousseau JL (1999) Intensive short term therapy with granulocyte-macrophage-colony stimulating factor support, similar to therapy for acute myeloblastic leukemia, does not improve overall results for adults with acute lymphoblastic leukemia. GOELAMS Group. Cancer 86(8):1496–1505
Nemunaitis J, Rosenfeld CS, Ash R, Freedman MH, Deeg HJ, Appelbaum F, Singer JW, Flomenberg N, Dalton W, Elfenbein GJ et al (1995) Phase III randomized, double-blind placebo-controlled trial of rhGM-CSF following allogeneic bone marrow transplantation. Bone Marrow Transplant 15(6):949–954
Gordon B, Spadinger A, Hodges E, Ruby E, Stanley R, Coccia P (1994) Effect of granulocyte-macrophage colony-stimulating factor on oral mucositis after hematopoietic stem-cell transplantation. Journal of Clinical Oncology: Official Journal of the Am Soc Clin Oncol 12(9):1917–1922
Rovirosa A, Ferre J, Biete A (1998) Granulocyte macrophage-colony-stimulating factor mouthwashes heal oral ulcers during head and neck radiotherapy. Int J Radiat Oncol Biol Phys 41(4):747–754
Bez C, Demarosi F, Sardella A, Lodi G, Bertolli VG, Annaloro C, Rimondini L, Porter SR, Carrassi A (1999) GM-CSF mouthrinses in the treatment of severe oral mucositis: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 88(3):311–315
Valcarcel D, Sanz MA Jr, Sureda A, Sala M, Munoz L, Subira M, Laborda R, Clopes A, Sierra J (2002) Mouth-washings with recombinant human granulocyte-macrophage colony stimulating factor (rhGM-CSF) do not improve grade III–IV oropharyngeal mucositis (OM) in patients with hematological malignancies undergoing stem cell transplantation. Results of a randomized double-blind placebo-controlled study. Bone Marrow Transplant 29(9):783–787
Ibrahim EM, al-Mulhim FA (1997) Effect of granulocyte-macrophage colony-stimulating factor on chemotherapy-induced oral mucositis in non-neutropenic cancer patients. Med Oncol 14(1):47–51
Hejna M, Kostler WJ, Raderer M, Steger GG, Brodowicz T, Scheithauer W, Wiltschke C, Zielinski CC (2001) Decrease of duration and symptoms in chemotherapy-induced oral mucositis by topical GM-CSF: results of a prospective randomised trial. Eur J Cancer 37(16):1994–2002
Rossi A, Rosati G, Colarusso D, Manzione L (2003) Subcutaneous granulocyte-macrophage colony-stimulating factor in mucositis induced by an adjuvant 5-fluorouracil plus leucovorin regimen. A phase II study and review of the literature. Oncology 64(4):353–360
Masucci G, Broman P, Kelly C, Lindahl S, Malmberg L, Reizenstein J, Alenius M, Lewensohn R (2005) Therapeutic efficacy by recombinant human granulocyte/monocyte-colony stimulating factor on mucositis occurring in patients with oral and oropharynx tumors treated with curative radiotherapy: a multicenter open randomized phase III study. Med Oncol 22(3):247–256
Clarkson JE, Worthington HV, Furness S, McCabe M, Khalid T, Meyer S (2010) Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev (8):CD001973. doi:10.1002/14651858.CD001973.pub4
Foncuberta MC, Cagnoni PJ, Brandts CH, Mandanas R, Fields K, Derigs HG, Reed E, Sonis ST, Fay J, LeVeque F, Pouillart P, Schrezenmeier H, Emmons R, Thiel E (2001) Topical transforming growth factor-beta3 in the prevention or alleviation of chemotherapy-induced oral mucositis in patients with lymphomas or solid tumors. J Immunother 24(4):384–388
Wymenga AN, van der Graaf WT, Hofstra LS, Spijkervet FK, Timens W, Timmer-Bosscha H, Sluiter WJ, van Buuren AH, Mulder NH, de Vries EG (1999) Phase I study of transforming growth factor-beta3 mouthwashes for prevention of chemotherapy-induced mucositis. Clin Cancer Res 6:1363–1380
de Koning BA, Philipsen-Geerling B, Hoijer M, Hählen K, Büller HA, Pieters R (2007) Protection against chemotherapy induced mucositis by TGF-beta(2) in childhood cancer patients: results from a randomized cross-over study. Pediatr Blood Cancer 48(5):532–539
Prince HM, Regester G, Gates P, Jablonskis L, Seymour JF, Lillie K, West R, Wolf M, Januszewicz H, Belford D (2005) A phase Ib clinical trial of PV701, a milk-derived protein extract, for the prevention and treatment of oral mucositis in patients undergoing high-dose BEAM chemotherapy. Biol Blood Marrow Transplant 11(7):512–520
Girdler NM, McGurk M, Aqual S, Prince M (1995) The effect of epidermal growth factor mouthwash on cytotoxic-induced oral ulceration. A phase I clinical trial. Am J Clin Oncol 18(5):403–406
Hong JP, Lee SW, Song SY, Ahn SD, Shin SS, Choi EK, Kim JH (2009) Recombinant human epidermal growth factor treatment of radiation-induced severe oral mucositis in patients with head and neck malignancies. Eur J Cancer Care (Engl) 18(6):636–641
Wu HG, Song SY, Kim YS, Oh YT, Lee CG, Keum KC, Ahn YC, Lee SW (2009) Therapeutic effect of recombinant human epidermal growth factor (RhEGF) on mucositis in patients undergoing radiotherapy, with or without chemotherapy, for head and neck cancer: a double-blind placebo-controlled prospective phase 2 multi-institutional clinical trial. Cancer 115(16)
Antin JH, Lee SJ, Neuberg D, Alyea E, Soiffer RJ, Sonis S, Ferrara JL (2002) A phase I/II double-blind, placebo-controlled study of recombinant human interleukin-11 for mucositis and acute GVHD prevention in allogeneic stem cell transplantation. Bone Marrow Transplant 29(5):373–377
Hunter A, Mahendra P, Wilson K, Fields P, Cook G, Peniket A, Crawley C, Hickling R, Marcus R (2009) Treatment of oral mucositis after peripheral blood SCT with ATL-104 mouthwash: results from a randomized, double-blind, placebo-controlled trial. Bone Marrow Transplant 43(7):563–569
Peterson DE, Barker NP, Akhmadullina LI, Rodionova I, Sherman NZ, Davidenko IS, Rakovskaya GN, Gotovkin EA, Shinkarev SA, Kopp MV, Kulikov EP, Moiseyenko VM, Gertner JM, Firsov I, Tuleneva T, Yarosh A, Woon CW (2009) Phase II, randomized, double-blind, placebo-controlled study of recombinant human intestinal trefoil factor oral spray for prevention of oral mucositis in patients with colorectal cancer who are receiving fluorouracil-based chemotherapy. J Clin Oncol 27(26):4333–4338
Levine JE, Blazar BR, DeFor T, Ferrara JL, Weisdorf DJ (2008) Long-term follow-up of a phase I/II randomized, placebo-controlled trial of palifermin to prevent graft-versus-host disease (GVHD) after related donor allogeneic hematopoietic cell transplantation (HCT). Biol Blood Marrow Transplant 14(9):1017–1021
Goldberg JD, Zheng J, Castro-Malaspina H, Jakubowski AA, Heller G, van den Brink MR, Perales MA (2012) Palifermin is efficacious in recipients of TBI-based but not chemotherapy-based allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant. doi:10.1038/bmt.2012.115 [Epub ahead of print]
Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG, Berger D (2011) Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. Journal of clinical oncology: official journal of the Am Soc Clin Oncol 29(20):2815–2820
Le QT, Kim HE, Schneider CJ, Murakozy G, Skladowski K, Reinisch S, Chen Y, Hickey M, Mo M, Chen MG, Berger D, Lizambri R, Henke M (2011) Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. Journal of clinical oncology: official journal of the Am Soc Clin Oncol 29(20):2808–2814
Caluwaerts S, Vandenbroucke K, Steidler L, Neirynck S, Vanhoenacker P, Corveleyn S, Watkins B, Sonis S, Coulie B, Rottiers P (2010) AG013, a mouth rinse formulation of Lactococcus lactis secreting human Trefoil Factor 1, provides a safe and efficacious therapeutic tool for treating oral mucositis. Oral Oncol 46(7):564–570
Zheng C, Cotrim AP, Sunshine AN, Sugito T, Liu L, Sowers A, Mitchell JB, Baum BJ (2009) Prevention of radiation-induced oral mucositis after adenoviral vector-mediated transfer of the keratinocyte growth factor cDNA to mouse submandibular glands. Clin Cancer Res 15(14):4641–4648
Acknowledgments
We acknowledge Professor Stephen Sonis for providing helpful suggestions.
Disclosures
The Mucositis Guidelines Update was sponsored by Helsinn Healthcare S.A., Switzerland and BioAlliance Pharma, France. Per MASCC policy, no industry representatives had any role in the development of the guidelines.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Raber-Durlacher, J.E., von Bültzingslöwen, I., Logan, R.M. et al. Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients. Support Care Cancer 21, 343–355 (2013). https://doi.org/10.1007/s00520-012-1594-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1594-5