Abstract
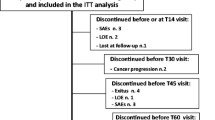
Tramadol is commonly used as second step drug of the analgesic ladder. In circumstances where the oral route is unavailable, rectal administration of opioids might be a simple alternative. The aim of this study was to compare the analgesic activity and tolerability of tramadol by oral and rectal administration in a double-blind, double-dummy crossover trial. The study included 60 cancer patients with cancer pain no longer responsive to non-opioid drugs. Each patient initially received oral tramadol 50 mg (drops), followed by tramadol sustained release 100 mg orally, and placebo rectally, or tramadol 100 mg rectally and placebo orally, twice a day, in a randomized sequence, on each of 3 days. Patients were allowed to take 50 mg of oral tramadol by drops as needed (four doses per day, to a maximum of 400 mg/day, including the basal dose given by the oral or rectal route). Pain intensity and relief and symptom scores were recorded every day and at the end of each phase of the crossover. The mean age of the patients was 66.1 years (SD 13.5 years); 36 were female, and 44 completed both periods. Patients dropped out due to adverse effects (15 patients) and refusal (1 patient). No differences in the use of rescue dose of oral tramadol were observed between the groups. No differences in pain intensity and relief scores, or in other symptoms between the two treatments were observed. No differences in treatment efficacy as judged by the clinician (P=0.73), in patient compliance (P=0.35), or in patient satisfaction regarding treatment (P<0.35) were found. No differences in adverse effects were found between the two treatments (25.5%, 13 patients, and 20.4%, 11 patients, with oral and rectal treatment, respectively). The proportion of preferences favored oral administration for both physicians (P=0.0002) and patients (P=0.002). Rectal administration of tramadol appears a reliable, noninvasive alternative method of pain control for patients no longer responsive to non-opioid analgesics, unable to take oral tramadol.

Similar content being viewed by others
References
Babul N, Provencher L, Laberge F, Harsanyi Z, Moulin D (1998) Comparative efficacy and safety of controlled-release morphine suppositories and tablets in cancer pain. J Clin Pharmacol 38:74–81
Bruera E, Faisinger R, Spachinsky K, Suarez-Almazor M, Inturrisi C (1995) Clinical efficacy and safety of a novel controlled-release morphine suppository and subcutaneous morphine in cancer pain: a randomized evaluation. J Clin Oncol 13:1520–1527
Cole L, Hanning C.D (1990) Review of the rectal use of opioids. J Pain Symptom Manage 5:118–126
Cossmann M, Wilsmann KM (1987) Effects and side effects of tramadol. An open phase IV study with 7198 patients. Therapiewocke 37:3475–3485
De Boer AG, De Leede LG, Breimer DD (1984) Drug absorption by sublingual and rectal route. Br J Anaesth 56:69–82
De Conno F, Ripamonti C, Saita L, MacEachern T, Hanson J, Bruera E (1995) Role of rectal route in treating cancer pain: a randomized crossover clinical trial of oral versus rectal morphine administration in opioid-naive cancer patients with pain. J Clin Oncol 13:1004–1008
Desmeules JA (2000) The tramadol option. Eur J Pain 4 [Suppl A]:15–21
Grond S, Radbruch L, Meuser T, Loick G, Sabatowski R, Lehmann KA (1999) High-dose tramadol in comparison to low-dose morphine for cancer pain relief. J Pain Symptom Manage 18:174–179
Jadad AR, Browman GP (1995) The WHO analgesic ladder for cancer pain management. JAMA 274:1870–1873
Jarlbaek L, Andersen M, Kragstrup J, Hallas J (2004) Cancer patients’ share in a population’s use of opioids. A linkage study between a prescription database and the Danish cancer registry. J Pain Symptom Manage 27:36–43
Leow K, Smith M, Williams B, Cramond T (1992) Single-dose and steady-state pharmacokinetics and pharmacodynamics of oxycodone in patients with cancer. Clin Pharmacol Ther 52:487–495
Leow KP, Cramond T, Smith MT (1995) Pharmacokinetics and pharmacodynamics of oxycodone when given intravenously and rectally to adult patients with cancer pain. Anesth Analg 80:296–302
Lintz W, Barth H, Osterloh G, Schmidt-Bothelt E (1998) Pharmacokinetics of tramadol and bioavailability of enteral tramadol formulations. Arzneimittelforschung 48:889–899
Lintz W, Barth H, Becker R, Frankus E, Schmidt-Bothelt E (1998) Pharmacokinetics of tramadol and bioavailability of enteral tramadol formulations. 2nd communication: drops with ethanol. Arzneimittelforschung 48:436–445
Mercadante S (1999) Treatment and outcome of cancer pain in advanced cancer patients followed at home. Cancer 85:1849–1858
Petrone D, Kamin M, Olson W (1999) Slowing the titration rate of tramadol HCl reduces the incidence of discontinuation due to nausea and/or vomiting: a double-blind randomized trial. J Clin Pharm Ther 24:115–123
Petzke F, Radbruch L, Sabatowski R, Karthaus M, Mertens A (2001) Slow-release tramadol for treatment of chronic malignant pain—an open multicenter trial. Support Care Cancer 9:48–54
Ripamonti C, Zecca E, Brunelli C, et al (1995) Rectal methadone in cancer patients with pain. A preliminary clinical and pharmacokinetic study. Ann Oncol 6:841–843
Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F (1987) A validation study of the WHO method for cancer pain relief. Cancer 59:850–856
Warren DE (1996) Practical use of rectal medications in palliative care. J Pain Symptom Manage 11:378–387
Zech DFJ, Grond S, Lynch J, Hertel D, Lehmann KA (1995) Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain 63:65–76
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mercadante, S., Arcuri, E., Fusco, F. et al. Randomized double-blind, double-dummy crossover clinical trial of oral tramadol versus rectal tramadol administration in opioid-naive cancer patients with pain. Support Care Cancer 13, 702–707 (2005). https://doi.org/10.1007/s00520-004-0760-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-004-0760-9