Abstract
Goals
Our goal was to evaluate the efficacy and tolerability of the Reliefband as an adjunct to standard antiemetics in patients receiving moderately-high to highly emetogenic chemotherapy.
Patients and methods
Forty-nine adult cancer patients receiving moderately-high or highly emetogenic chemotherapy were randomized to receive either the active Reliefband (n=26) or an inactive device (n=23). Patients continued to receive all scheduled and as needed antiemetic agents as prescribed. The device was worn the day of chemotherapy administration for 5 days (days 1–5). Patients maintained a daily dairy of nausea severity, vomiting and retching episodes, and antiemetic medications taken. Each patient completed a Functional Living Index Emesis (FLIE) and a tolerability survey at the conclusion of the study. A Wilcoxon rank sum test was used to compare the number of vomiting episodes, severity of nausea and FLIE scores between the two groups.
Main results
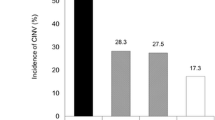
Patients wearing the active Relifband experienced less vomiting (Reliefband 1.9 versus inactive device 4.6 mean episodes; p=0.05), retching (1.4 versus 3.6 mean episodes; p=0.05), and nausea severity (0.91 versus 1.65 mean cm/day; p=0.01) over the 5-day period compared to patients wearing the inactive device. Vomiting was statistically significantly reduced during the delayed period (0.42 versus 1; p=0.032), whereas nausea was significantly reduced during the acute (0.71 versus 2.3; p=0.028) and delayed (1.8 versus 3.3; p=0.020) periods. FLIE scores did not differ between the two treatment groups (91 versus 80; p=0.088).
Conclusions
This study suggests that patients receiving moderately-high to highly emetogenic chemotherapy who experience nausea and vomiting despite scheduled antiemetics may benefit from the use of the Reliefband as an adjunct to antiemetics. Limitations of this study include differences in risk factors for emesis, chemotherapy, and antiemetic regimens. A larger, better, controlled randomized study is needed to better define optimal use of this device.
Similar content being viewed by others
References
Abang AM, Takemoto MH, Pham T, Mandanas RA, Roy V, Selby GB, et al (2000) Efficacy and safety of oral granisetron versus i.v. granisetron in patients undergoing peripheral blood progenitor cell and bone marrow transplantation. Anticancer Drugs 11:(2)137–142
Anonymous (1995) Ondansetron versus granisetron, both combined with dexamethasone, in the prevention of cisplatin-induced emesis. Italian Group of Antiemetic Research. Ann Oncol 6:805–810
Anonymous (1999) ASHP Therapeutic guidelines on the pharmacologic management of nausea and vomiting in adult and pediatric patients receiving chemotherapy or radiation therapy or undergoing surgery. Am J Healthsystem Pharm 56:(8)729–764
Birch R, Weaver CH, Carson K, Buckner CD (1998) A randomized trial of once vs twice daily administration of intravenous granisetron with dexamethosone in patients receiving high-dose cyclophosphamide, thiotepa and carboplatin. Bone 22:(7)685–8
Bshunow PW, Matteson SE, Morrow G, Roscoe J (2002) Patients with intractable chemotherapy-induced nausea find acustimulation band helpful. Procedings of the annual meeting, Am Soc Clin Oncol 22: Abstract
Campos D, Pereira JR, Reinhardt RR, Carracedo C, Poli S, Vogel C, et al (2001) Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol 15 19:(6)1759–1767
Cocquyt V, Van Belle S, Reinhardt RR, Decramer ML, O'Brien M, Schellens JH et al (2001) Comparison of L-758,298, a prodrug for the selective neurokinin-1 antagonist, L-754,030, with ondansetron for the prevention of cisplatin-induced emesis. Eur J Cancer 37:835–842
Dundee JW. (1990) Belfast experience with P6 acupuncture antiemesis. Ulster Med J 59:63–70
Dundee JW, Ghaly RG, Fitzpatrick KT, Abram WP, Lynch GA. (1989) Acupuncture prophylaxis of cancer chemotherapy-induced sickness. J R Soc Med 82:268–271
Dundee JW, McMillan CM (1990) Clinical uses of P6 acupuncture antiemesis. Acupunct Electrother Res 15:211–215
Dundee JW, Yang J (1990) Prolongation of the antiemetic action of P6 acupuncture by acupressure in patients having cancer chemotherapy. J R Soc Med 83:360–362
Dundee JW, Yang J, Ghaly RG (1990) Vomiting and chemotherapy. Lancet 335:541
Dundee JW, Yang J, McMillan C (1991) Non-invasive stimulation of the P6 (Neiguan) antiemetic acupuncture point in cancer chemotherapy. J R Soc Med 84:210–212
Dranitsaris G, Leung P, Ciotti R, Ortega A, Spinthouri M, Liaropoulos L, et al (2001) A multinational study to measure the value that patients with cancer place on improved emesis control following cisplatin chemotherapy. Pharmacoeconomics 19(9):955–67
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, et al (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. [erratum appears in J Clin Oncol 1999 Dec;17(12):3860; J Clin Oncol 2000 Aug;18(16):3064]. J Clin Oncol 17:2971–2994
Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, et al (1996) On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Onc 7:(2)189–195
Grunberg SM, Boutin N, Ireland A, Miner S, Silveira J, Ashikaga T (1996) Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer 4:(6)435–439
Herrington JD, Kwan P, Young RR, Lagow E, Lagrone L, Riggs MW (2000) Randomized, multicenter comparison of oral granisetron and oral ondansetron for emetogenic chemotherapy. Pharmacotherapy 20:1318–1323
Hesketh PJ, Gralla RJ, Webb RT, Ueno W, DelPrete S, Bachinsky ME, et al (1999) Randomized phase II study of the neurokinin 1 receptor antagonist CJ-11, 974 in the control of cisplatin-induced emesis. J Clin Oncol 17:(1)338–343
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, et al (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15:103–109
Pearson J, Blumentals W, Martin A, Carides A, Horgan K, Wittreich J, et al (2000) Validation of Functional Living Index-Emesis (FLIE) Quality-of-life questionnaire for acute and delayed chemotherapy-induced emesis. Proceedings of the annual meeting, Am Soc Clin Oncol 20:2384
Laszlo J (1983) Nausea and vomiting as major complications of cancer chemotherapy. Drugs [Suppl 1] 25:1–7
Lindley CM, Hirsch JD, O'Neill CV, Transau MC, Gilbert CS, Osterhaus JT (1992) Quality of life consequences of chemotherapy-induced emesis. Qual Life Res 1:331–340
Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A, et al (1999) Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754, 030 Antiemetic Trials Group. New Eng J Med 340:(3)190–195
Orchard PJ, Rogosheske J, Burns L, Rydholm N, Larson H, DeFor TE, et al (1999) A prospective randomized trial of the anti-emetic efficacy of ondansetron and granisetron during bone marrow transplantation. Biol Blood Marrow Transplant 5:(6)386–393
Osoba D, Zee B, Warr D, Latreille J, Kaizer L, Pater J (1997) Effect of postchemotherapy nausea and vomiting on health-related quality of life. The quality of life and symptom control committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer 5:(4)307–313
Osowski CL, Dix SP, Lynn M, Davidson T, Cohen L, Miyahara T, et al (1998) An open-label dose comparison study of ondansetron for the prevention of emesis associated with chemotherapy prior to bone marrow transplantation. Support Care Cancer 6:511–517
Oyama H, Kaneda M, Katsumata N, Akechi T, Ohsuga M. (2000) Using the bedside wellness system during chemotherapy decreases fatigue and emesis in cancer patients. J Med Syst 24:173–182
Pearl ML, Fischer M, McCauley DL, Valea FA, Chalas E. (1999) Transcutaneous electrical nerve stimulation as an adjunct for controlling chemotherapy-induced nausea and vomiting in gynecologic oncology patients. Cancer Nurs 22:307–311
Saller R, Hellenbrecht D, Buhring M, Hess H. (1986) Enhancement of the antiemetic action of metoclopramide against cisplatin-induced emesis by transdermal electrical nerve stimulation. J Clin Pharmacol 26:115–119
Sigsgaard T, Herrstedt J, Handberg J, Kjaer M, Dombernowsky P (2001) Ondansetron plus metopimazine compared with ondansetron plus metopimazine plus prednisolone as antiemetic prophylaxis in patients receiving multiple cycles of moderately emetogenic chemotherapy. J Clin Onc 19:(7)2091–2097
Vincent CA, Richardson PH (1986) The evaluation of therapeutic acupuncture: concepts and methods. Pain. 24:(1)1–13
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by Woodside Biomedical, Inc., Carlsbad, California USA.
Rights and permissions
About this article
Cite this article
Treish, I., Shord, S., Valgus, J. et al. Randomized double-blind study of the Reliefband as an adjunct to standard antiemetics in patients receiving moderately-high to highly emetogenic chemotherapy. Support Care Cancer 11, 516–521 (2003). https://doi.org/10.1007/s00520-003-0467-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-003-0467-3