Summary
Aim
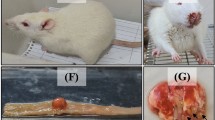
The aim of the present study was to evaluate the effect of crizotinib on visceral organs in an experimental rat model.
Methods
Eighteen Wistar albino rats were divided into three groups: experimental toxicity was induced with crizotinib (10 mg/kg) administered for 28 days (Group 1), 42 days (Group 2) orally by gavage. Control group received only distilled water. Rats in Group 1 and Group 2 were sacrificed after the collection of blood and tissue samples on the 28th and 42nd days, respectively.
Results
Subjects in Group 1 and Group 2 had abnormal histology mainly in lung and liver. There were intraalveolar hemorrhage in lungs; mild portal inflammation, perivenular focal and confluent necrosis in liver; inflammatory reaction in renal pelvis and periureteral areas, and focal pancreatitis in pancreas.
Conclusion
This study is the first to evaluate the histopathological features of toxicity of crizotinib in a rat model.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Collins LG, Haines C, Perkel R, et al. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75(1):56–63.
Alberts WM. Diagnosis and management of lung cancer executive summary: ACCP evidence-based clinical practice guidelines (2nd Edition). Chest. 2007;132(3 Suppl):1S–19S.
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39.
Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500.
Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703.
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6.
Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942–6.
Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9.
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Stahel RA, Rosell R, Thatcher N, Soria JC. ALK translocation and crizotinib in non-small cell lung cancer: an evolving paradigm in oncology drug development. Eur J Cancer. 2012;48(7):961–73.
Crinò L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011;29 (suppl; abstr 7514).
Smith EB, Goulet L, Walker GS, et al. Metabolism and excretion of Crizotinib in rats and dogs. 17th North American Regional International society for the study of xenobiotics Meeting. (P82).
Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–70.
Qian H, Gao F, Wang H, et al. The efficacy and safety of crizotinib in the treatment of anaplastic lymphoma kinase-positive non-small cell lung cancer: a meta-analysis of clinical trials. BMC Cancer. 2014;14:683.
Loriot Y, Perlemuter G, Malka D, et al. Drug insight: gastrointestinal and hepatic adverse effects of molecular-targeted agents in cancer therapy. Nat Clin Pract Oncol. 2008;5(5):268–78.
Demirci U, Buyukberber S, Yılmaz G, et al. Hepatotoxicity associated with lapatinib in an experimental rat model. Eur J Cancer. 2012;48(2):279–85.
Ripault MP, Pinzani V, Fayolle V, et al. Crizotinib-induced acute hepatitis: first case with relapse after reintroduction with reduced dose. Clin Res Hepatol Gastroenterol. 2013;37(1):e21–3.
Sato Y, Fujimoto D, Shibata Y, et al. Fulminant hepatitis following crizotinib administration for ALK-positive non-small-cell lung carcinoma. Jpn J Clin Oncol. 2014;44(9):872–5.
Pariente A, Etcharry F, Cales V, et al. Imatinib mesylate-induced acute hepatitis in a patient treated for gastrointestinal stromal tumour. Eur J Gastroenterol Hepatol. 2006;18(7):785–7.
Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99(7):2310–4.
Ono A, Takahashi T, Oishi T, et al. Acute lung injury with alveolar hemorrhage as adverse drug reaction related to crizotinib. J Clin Oncol. 2013;31(26):e417–9.
Muller NL, White DA, Jiang H, et al. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer. 2004;91:S24–S30.
Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–56.
Higenbottam T, Kuwano K, Nemery B, et al. Understanding the mechanisms of drug-associated interstitial lung disease. Br J Cancer. 2004;91(Suppl 2):S31–7.
Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361(9352):137–9.
Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120(2):617–24.
Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology. 2013;13(5):461–7.
Rothenstein JM, Letarte N. Managing treatment-related adverse events associated with Alk inhibitors. Curr Oncol. 2014;21(1):19–26.
Martín Martorell P, Huerta Alvaro M, Solís Salguero MA, et al. Crizotinib and renal insufficiency: a case report and review of the literature. Lung Cancer. 2014;84(3):310–3.
Brosnan EM, Weickhardt AJ, Lu X, et al. Drug-induced reduction in estimated glomerular filtration rate in patients with ALK-positive non-small cell lung cancer treated with the ALK inhibitor crizotinib. Cancer. 2014;120(5):664–74.
Conflict of interest
O. Gumusay, G. Esendagli-Yilmaz, A. Uner, B. Cetin, S. Buyukberber, M. Benekli, M. N. Ilhan, U. Coskun, O. Gulbahar, and A. Ozet declare that there are no actual or potential conflicts of interest in relation to this article. The authors received no additional financial support or national funding for this study. There is no substantial contribution provided by a person different from the authors. This submission has not been published anywhere previously and that it is not simultaneously being considered for any other publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gumusay, O., Esendagli-Yilmaz, G., Uner, A. et al. Crizotinib-induced toxicity in an experimental rat model. Wien Klin Wochenschr 128, 435–441 (2016). https://doi.org/10.1007/s00508-016-0984-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-016-0984-y