Summary
Background
Viral lower respiratory tract infections are the leading cause of hospitalizations in preschool children. Clinical pictures of different viral causes are not well characterized. The aim of this study was to establish the differences in clinical and laboratory characteristics between the different viral causes of lower respiratory tract infections in preschool children.
Methods
We included 278 preschool children hospitalized because of lower respiratory tract infection. White blood cell count and C-reactive protein values were determined and chest X-ray was performed in most patients. Polymerase chain reaction assay was used for the detection of viral pathogens from nasopharyngeal swab.
Results
Pneumonia was present in 71.4 % of all coronavirus infections, 35.1 % of all respiratory syncytial virus infections, and 13.0 % of all rhinovirus infections. Coronavirus (p = 0.03) and respiratory syncytial virus (p < 0.01) were retrospectively shown to be associated with the presence of pneumonia and rhinovirus (p < 0.01) with the absence of pneumonia. Wheezing was present in 81.5 % of all rhinovirus infections and in only 33.3 % of all adenovirus infections. Rhinovirus (p < 0.01) was associated with the presence of wheezing and adenovirus (p = 0.05) with the absence of wheezing. In adenovirus infections mean C-reactive protein value was 72.4 mg/L and white blood cell count 19.000/µl, both significantly higher than in other viruses (p < 0.01).
Conclusions
Clinical and laboratory characteristics of viral lower respiratory tract infections significantly differ. With the advance of viral detection methods and increase of knowledge it becomes possible to characterize different respiratory viral infections and to improve the differential diagnosis.
Similar content being viewed by others
Introduction
Lower respiratory tract infections (LRTIs) are the leading cause of global morbidity and mortality in preschool children and major cause of hospital admissions in young children in the developed world. They also represent a substantial burden on health services [1, 2]. LRTIs can be clinically broadly divided into tracheitis, pneumonia, bronchitis, and bronchiolitis. However, the clinical pictures overlap. Furthermore, some upper respiratory tract infections (URTIs) provoke wheezing in susceptible children even without lower airway infection, which brings additional complexity [3]. In small children LRTIs generally have viral etiology [4]. The most common cause of LRTI is the respiratory syncytial virus (RSV); other possible viral pathogens are human rhinovirus, parainfluenza virus, human metapneumovirus, influenza virus, adenovirus, coronavirus, and human bocavirus. Viral LRTI cannot be reliably distinguished from an infection with Mycoplasma pneumonia [5, 6].
Viruses also account for the largest proportion of childhood pneumonia [7]. RSV is believed to be the most important viral pathogen causing pneumonia in young children [8, 9]. Other common causes of viral pneumonia in children are adenovirus, influenza, and parainfluenza virus. The current epidemiological situation, patient’s age, clinical symptoms, radiographic and laboratory data, and response to treatment can help to differentiate viral from bacterial pneumonia. However, no clinical algorithm exists that will distinguish clearly between the different causes of childhood pneumonia [4]. Although chest radiographs and laboratory studies cannot reliably distinguish between different viral pathogens and even not between viral and bacterial etiology, they may be necessary to assess the severity of illness, evaluate potential complications, or to exclude other conditions in the differential diagnosis [10, 11]. Accurate and rapid diagnostic tests that establish the cause of LRTIs in children have the potential to reduce the use of antibiotics, to improve their targeted use and help to control the nosocomial transmission [3].
Until recently, the dominant standpoint was that LRTIs caused by RSV, parainfluenza, adenovirus, rhinovirus, metapneumovirus, coronavirus, and bocavirus are clinically indistinguishable [3]. With the advance and availability of accurate respiratory pathogen detection, it becomes possible to characterize the clinical pictures of individual respiratory pathogens and to delineate the differences in clinical and laboratory features between them. The aim of our study was to find possible associations of particular viral LRTI with clinical signs and basic laboratory results. These findings could lead to more accurate clinical judgment in primary health care and other conditions of limited diagnostics. Our hypothesis was that particular respiratory viruses cause different clinical pictures and/or basic laboratory results.
Patients, materials, and methods
In our retrospective study, we included all otherwise healthy preschool children hospitalized at Clinic of Pediatrics, University Medical Centre Maribor, from 1 January 2012 to 31 October 2013 because of signs or symptoms of LRTI and data were collected from their medical records. Despite the retrospective nature of the study we got informed consent for blood withdrawal and all the procedures from parents or caregivers, as we usually do for all of our hospitalized patients. Children were treated under the diagnosis of pneumonia, bronchitis, or bronchiolitis. Children with croup were not included. We excluded all premature infants, children with cancer, immunodeficiency, transplantation, known asthma or other chronic respiratory (e.g., mucoviscidosis), heart or neurologic diseases. We also excluded all children with invasive bacterial (super) infections (e.g., bacterial pneumonia, bacteriemia, and purulent meningitis). Therefore we also excluded the patients with lobar pneumonia. Altogether 278 children with at least one clinical and/or X-ray sign of LRTI were included. Wheezing, pharyngitis, and rhinitis was diagnosed clinically by the pediatrician, who treated the child. Pneumonia was diagnosed by the chest X-ray, confirmed by radiologist and pediatrician. Chest X-ray was performed only in those 152 patients who had clinical signs or symptoms suggestive of pneumonia (e.g., tachypnea, respiratory distress, crackles, and other localized auscultatory findings). Patient was considered febrile, when the body temperature at any time during the treatment exceeded 38 ℃, measured with an infrared tympanic thermometer. Venous blood was taken during the routine procedure in all treated children for the analysis of complete and differential blood count and C-reactive protein (CRP). In some cases laboratory tests were performed more than once and we considered the highest CRP value and white blood cell (WBC) count for statistical analysis. Children were treated with supplemental oxygen, administered via nasal prongs or face mask when oxygen saturation of peripheral blood (SpO2), measured by pulse oxymeter fell below 92 % or when the signs of respiratory distress (at the discretion of physician) were present. Patients who needed ventilatory support were treated at our intensive care unit (ICU) and were all endotracheally intubated. Criteria for the ICU admission were: hypoxemia (arterial oxygen tension < 60 mm Hg or SpO2 < 92 %) persisting despite administration of more than 40 % oxygen, persisting hypercapnia (carbon dioxide tension over 50 mm Hg), apnea, persistent respiratory acidosis, worsening respiratory distress, and disturbed mental status. All the patients treated in PICU were enrolled in the study when they were first admitted to normal ward. Clinical and laboratory characteristics analyzed in this study also refer to this (first) part of the hospitalization.
Detection of respiratory pathogens
For the detection of a causative agent, nasopharyngeal swab (Copan Diagnostics, Murrieta, California, USA) was collected at admission in all patients. The sampling method is noninvasive and user-friendly and allows sampling without causing major discomfort. Collected swabs were stored in 2 ml of 0.9 % sterile salt solution and kept at 4 ℃ until processed or for maximum of 3 days. Microbiological testing was performed at the National Laboratory for Health, Environment and Food, Maribor. In order to encompass the majority of described respiratory pathogens in sample material, multiplex PCR assay was used [12]. The FilmArray Respiratory Panel (RP) (BioFire Diagnostics, Salt Lake City, Utah, USA) is FDA approved and CE-IVD certified. It detects influenza A virus (subtype H1, H1N1 and H3), influenza B virus, RSV, metapneumovirus, coronaviruses (subtypes NL63, OC43, 229E and HKU1), adenovirus, parainfluenza viruses (serotype 1–4), bocavirus, rhinovirus, enterovirus, Bordetella pertussis, Mycoplasma pneumoniae, and Chlamydophila pneumoniae. The assay also includes internal controls for amplification and extraction. In this study we used FilmArray RP IVD v1.6 pouches. The sample was added to the pouch in microbiological safety cabinet class II according to the manufacturer’s protocol. In short, the FilmArray pouch stores all of the necessary reagents for sample preparation, RT–PCR, and detection in a freeze–dried format. Approximately 1 ml of hydration solution and 300 μl of 1:1.5 diluted sample in buffer was injected into the pouch.
Statistical analysis
Data analysis was carried out using SPSS version 19.0 (SPSS, Chicago, IL, USA). The two-sided Fisher’s exact test was used to calculate the significance of differences in frequencies, odds ratio (OR), and 95 % confidence interval (CI) for clinical data which are categorical variables (presence of pneumonia, wheezing, fever, pharyngitis, rhinitis) between monoinfections and coinfections. Two-sided t-test for two independent samples was used to test the differences between monoinfections and coinfections in clinical and laboratory data which are continuous variables: duration of hospitalization, highest CRP value and WBC count.
Viruses such as influenza A, influenza B, and enteroviruses that were isolated in less than ten cases were omitted from further statistical analysis of association. The same applies also for Mycoplasma pneumoniae. All serotypes of parainfluenza virus and coronavirus were joined in one group for the purpose of statistical analysis. When testing only for association of monoinfections with clinical and laboratory characteristics, we also omitted data for coronavirus, adenovirus, and bocavirus infections, which occurred mostly as coinfections and caused less than five monoinfections cases. Chi-square test was used to test the significance of correlation between the causal agent and clinical data which are categorical variables and one-way ANOVA was used for continuous variables. We upgraded the statistical analysis with multiple logistic regression, adjusting for age and sex, which was used to test the significance of association between a particular virus and those clinical data which are categorical variables and generalized linear model was used for the same purpose for continuous variables. Values of p < 0.05 were considered significant.
Results
Demographic and clinical characteristics of patients
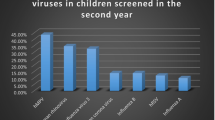
Out of 278 included patients (134 girls, 48.2 %), we detected at least one respiratory pathogen in 198 (71.2 %). Single microorganism was isolated in 131 (66.2 % of all positive) samples, in 55 (27.8 %) samples we proved double infection and in 12 (6.1 %) samples we identified three microorganisms (triple infection). There were altogether 277 isolates. The most common isolate was rhinovirus, followed by RSV, and bocavirus. Frequencies of detected viruses are presented in Fig. 1.
Frequencies of respiratory viruses detected in nasopharyngeal swab in preschool children hospitalized because of lower respiratory tract infection. HEV human enterovirus, FluB influenza B, FluA influenza A, hMPV human metapnevmovirus, HCoV human coronavirus, ADV adenovirus, PIV parainfluenza virus, HBOV bocavirus, RSV respiratory syncytial virus, HRV human rhinovirus, N number of cases with particular viral infection
Bocavirus, adenovirus, and coronavirus were only rarely encountered as the only isolate and were found as coinfection in 29 (87.8 % of all isolates), 14 (93.3 %), and 12 (85.7 %) of cases, respectively.
Most of the infections occurred during the four winter months. From December until March we identified 104 (52.5 %) cases and from May until August only 30 (15.1 %) cases of LRTIs. During the four winter months (December to March), 81.8 % of all RSV infections were detected. In January and February this virus was also the most commonly identified pathogen. Rhinovirus prevailed as an isolate from March to May and then again from September to November. Of all bocavirus infections 75.6 % were detected from November to April and 63.6 % of all metapneumovirus infections during the late spring (in May and June).
Demographic, clinical, and laboratory characteristics of patients are presented in Table 1.
Influence of coinfection on clinical and laboratory data
We first compared clinical and laboratory data between patients with monoinfection and coinfection, which is shown in Table 2.
Except for the presence of pharyngitis, which was more common in monoinfections there were no differences between both groups in other clinical and laboratory characteristics.
Clinical and laboratory characteristics of viral infections
Pneumonia
Pneumonia was present in 71.4 % (10 out of 14 cases) of coronavirus infections, 63.6 % (7 out of 11) of metapneumovirus infections, 40.9 % (9 out of 22) of parainfluenza infections, 35.1 % (27 out of 77) of RSV infections, 20.0 % (three out of 15) of adenovirus infections, 15.2 % (5 out of 33) of bocavirus infections, and 13.0 % (12 out of 92) of rhinovirus infections (p < 0.01).
When testing only monoinfections, pneumonia was present in 62.5 % (5 out of 8 cases) of metapneumovirus infections, 45.5 % (5 out of 11) of parainfluenza infections, 33.3 % (19 out of 51) of RSV infections and only in 8.3 % (4 out of 52) of rhinovirus infections (p < 0.01).
We also tested this association more specifically with multiple logistic regression model, where RSV (p < 0.01) and coronavirus (p = 0.03) infections were found to be significantly associated with pneumonia and rhinovirus infection was associated with the absence of pneumonia (p < 0.01).
Wheezing
Wheezing was present in 81.5 % (75 out of 92 cases) of rhinovirus infections, 60.6 % (20 out of 33) of bocavirus infections, 50.0 % (11 out of 22) of parainfluenza infections, 46.7 % (36 out of 77) of RSV infections, 36.4 % (4 out of 11) of metapneumovirus infections, 35.7 % (4 out of 14) of coronavirus infections, and 33.3 % (5 out of 15) of adenovirus infections (p < 0.01).
When testing only monoinfections, wheezing was present in 80.8 % (42 out of 52 cases) of rhinovirus infections, 45.5 % (5 out of 11) of parainfluenza infections, 37.5 % (3 out of eight) of metapneumovirus infections, and 35.3 % (18 out of 51) of RSV infections (p < 0.01).
According to multiple logistic regression model rhinovirus infection was specifically associated with wheezing (p < 0.01) and adenovirus infection with the absence of wheezing (p = 0.04).
Need for supplemental oxygen
Supplemental oxygen was needed in 50 % (7 out of 14 cases) of coronavirus infections, 48.1 % (37 out of 77) of RSV infections, 39.1 % (36 out of 92) of rhinovirus infections, 36.4 % (4 out of 11) of metapneumovirus infections, 30.3 % (10 out of 33) of bocavirus infections, 26.7 % (4 out of 15) of adenovirus infections, and 9.1 % (2 out of 22) of parainfluenza infections (p = 0.01).
When considering only monoinfections, additional oxygen was needed in 54.9 % (28 out of 51 cases) of RSV infections, 42.3 % (22 out of 52) of rhinovirus infections, 37.5 % (3 out of 8) of metapneumovirus infections, and 10 % (1 out of 10) of parainfluenza infections (p = 0.04).
According to multiple logistic regression analysis parainfluenza virus (p < 0.01) and bocavirus (p = 0.05) infections were associated with less common need for supplemental oxygen compared with other viruses.
Other characteristics
The comparison of other demographic, clinical, and laboratory characteristics between different viral causes of LTRIs is presented in Table 3. Viral infections differ significantly regarding the presence of fever. Considering only monoinfections (not shown in Table 3), fever was present in 75 % of metapneumovirus infections, 56.9 % of RSV infections, 54.5 % of parainfluenza infections, and 32.7 % of rhinovirus infections (p = 0.03). More specifically: rhinovirus infections were associated with the absence of fever (p = 0.01).
In addition we found significant differences in the age of patients, length of hospitalization, CRP level, and WBC count (p < 0.01) between different viral infections.
When tested with a generalized linear model adenovirus infections were specifically associated with higher CRP (p < 0.01), higher WBC (p < 0.01), and were older (p < 0.03); however, the length of hospitalization was shorter in adenovirus infections (p = 0.01). Rhinovirus infections were associated with higher WBC (p = 0.01) and shorter length of stay (p < 0.01). RSV infections were associated with lower CRP (p < 0.01), lower WBC (p < 0.01), and lower age (p < 0.01); however, the length of hospitalization was longer in RSV infections (p < 0.01). Duration of hospitalization was shorter in bocavirus infections (p < 0.01). Patients with metapneumovirus infections were older (p < 0.01).
Discussion
Longer length of hospitalization and association with pneumonia encountered in our patients with RSV infection both support the concept of more serious lower respiratory tract injury caused by RSV, compared to other viruses [8]. Human coronaviruses have been known to induce upper respiratory tract symptoms such as nasal congestion and rhinorrhea and their pathogenicity in healthy infants and children was considered to be low. Coronaviruses are often found even in asymptomatic children [13]. On the other hand, coronavirus infections have been detected in variable proportions (2–8 %) of neonates, infants, and young children hospitalized with community-acquired pneumonia [14]. In our study coronaviruses were mainly found as a coinfection, as already reported previously [15]. However, association with pneumonia and need for supplemental oxygen, both indicate high pathogenicity of the virus, which was recently reported also by Lee et al. [16]. Rhinoviruses may cause pneumonia in immunosuppressed patients, elderly persons, and children with cystic fibrosis; however, their true impact is questionable because they may be an inciting event for other infectious processes and are often detected even in asymptomatic children [17]. Rhinoviruses can also cause severe LRTIs in preterm infants and even in otherwise healthy children [18, 19]. Garcia-Garcia et al. [20] even found rhinovirus as a most common cause of viral pneumonia in otherwise healthy school children. Our results support the important role of human rhinoviruses in children, hospitalized because of the LRTI symptoms; however, they were not found to be a common cause of pneumonia. Shorter length of hospitalization and significant absence of fever in our patients with rhinovirus infections probably also reflect a less severe direct injury of the lower airways and/or prevailing mechanism of asthma exacerbation in rhinovirus infections, which is more amenable to treatment compared to direct LRT injury, typical for other viruses.
Adenoviruses are relatively common cause of viral pneumonia and may manifest with lobar infiltrates, high fever, leukocytosis, and high CRP—features more typical of bacterial diseases [21]. In different studies adenovirus infections caused between 7 and 12 % of all community acquired pneumonias [8, 22–24], which is considerably more compared to our results. Low prevalence of adenoviral pneumonia could be partially explained by lower sensitivity of FilmArray Respiratory Panel for the detection of several human adenovirus serotypes. However, only one out of missed serotypes is a frequent causative agent of respiratory disease [25], and pneumonia was detected in a small proportion of all adenovirus infections in our study. Shorter length of hospitalization with adenovirus probably also reflects milder forms of inflammation of lower airways, compared to other viruses. The total number of cases in our study caused by adenovirus was too small to be significant and most of them occurred as a coinfection which further diminishes the strength of any conclusions.
We found rhinoviruses to be the most common cause of wheezing in hospitalized children. Our results support the prevailing concept, which considers rhinovirus infection as the single most important trigger of asthma exacerbations in preschool children [26]. Wheezing was detected in only half of our RSV caused LRTI cases, which is less than is usually stated in the literature [27]. Adenoviral LRTIs were associated with the absence of wheezing as opposed to the prevailing concept according to which adenovirus-caused bronchiolitis is clinically indistinguishable from RSV and other viruses [5].
The course of infection with parainfluenza and bocavirus was relatively mild and with less need of supplemental oxygen compared to other viruses. Parainfluenza virus is a common cause of URTIs and croup. It also accounts for 20–40 % of lower respiratory tract illnesses (e.g., bronchiolitis, pneumonia) in children from which a virus is recoverable [28]. According to our results parainfluenza virus was a less common cause of LRTI.
We found bocavirus mostly as a coinfection, similar to some previous studies [29]. The high rate of coinfection may be due to the fact that bocavirus has been shown to have prolonged shedding even from asymptomatic children [29]. Therefore it is difficult to establish the clinical characteristics of bocavirus infection. The most consistently reported symptoms include cough, fever, and rhinorrhea [30]. Higher titers of bocavirus in respiratory secretions have been associated with increased wheezing, suggesting a causal role [31], which is consistent with our results.
Higher WBC count and CRP values found in our adenovirus caused LRTIs to support the previous findings [21]. Of all common viruses causing LRTIs, adenovirus is the least distinguishable from bacterial infections on the ground of laboratory results. More surprisingly, our rhinovirus infections were also associated with higher WBC count, confirming the results of Bicer et al., who found that rhinovirus and adenovirus infections were both associated with higher WBC count; however, analyzed together in LRTI and URTI. They also found longer length of hospitalization and younger age in patients with RSV infection which is another characteristic also found in our patients [32]. RSV infections in our study were characterized by lower WBC count and CRP values compared to other viruses, a feature not described previously [33], which must be confirmed on a larger sample.
On the other hand, viral infections in our study did not differ in the upper respiratory tract symptoms supporting the concept of overlapping clinical characteristics of different viral respiratory tract infections [5].
We found a similar percentage of coinfections as some previous studies [34] and our patients with coinfections had clinical pictures no more severe than monoinfections, an interesting finding already reported recently by Bicer et al. [32]. The lack of differences between monoinfections and coinfections may reflect one of the major limitations of our study; we cannot reliably distinguish between true viral infection and asymptomatic (or post-symptomatic) shedding. According to our results, we can speculate that asymptomatic shedding is more common than true coinfection. In different studies rhinovirus was isolated from nasopharynx in 0–45 % (mean 14 %) of healthy individuals, though PCR positivity usually lasted for less than 2 weeks [17, 35]. Furthermore, nasopharyngeal colonization does not necessarily reflect the infection of lower airways. However, as it is difficult to obtain adequate specimens from the lower respiratory tract, samples from nasopharynx remain the most accurate approximation.
Regarding the frequencies of particular viruses we found similar distribution as reported by others in children hospitalized because of acute respiratory tract infection [36, 37]. Slightly higher frequencies of adenovirus and influenza viruses in those studies probably reflect the inclusion of patients with URTI. Our study lasted a few months less than 2 years and certain fall and winter months were represented only once, which could distort the epidemiological data. As we included only the patients with LRTI who needed hospitalization, our results do not necessary reflect the whole spectrum of clinical and laboratory characteristics of studied viruses and certain viruses may have different prevalence in outpatient setting as recently reported by Hara et al. [39], who found higher prevalence of adenovirus (30.2 %) and metapneumovirus (16.1 %) in Japanese pediatric outpatients [38].
In conclusion we may say that clinical pictures of particular viruses causing LRTI differ more than it was supposed previously. With the advance of detection techniques and with the epidemiological studies on larger samples, it will become possible to distinguish between different causes of LRTI not only in the hospital setting but also in the primary care. Better characterization of different viral infections could diminish the rate of hospitalization and the use of antibiotics.
References
Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Etiology and epidemiology of childhood pneumonia. Bull World Health Organ. 2008;86(5):408–16.
Nair H, Simoes EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381(9875):1380–90.
Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011;52(4):284–9.
Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet. 2011;377(9773):1264–75.
Stempel HE, Martin ET, Kuypers J, Englund JA, Zerr DM. Multiple viral respiratory pathogens in children with bronchiolitis. Acta Paediat. 2009;98(1):123–6.
Coffin SE. Bronchiolitis: in-patient focus. Pediatr Clin North Am. 2005;52(4):1047–57.
Korppi M, Don M, Valent F, Canciani M. The value of clinical features in differentiating between viral, pneumococcal and atypical bacterial pneumonia in children. Acta Paediatr. 2008;97(7):943–7.
Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39(5):681–6.
Nascimento-Carvalho CM, Ribeiro CT, Cardoso MR, et al. The role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing country. Pediatr Infect Dis J. 2008;27(10):939–41.
Don M, Valent F, Korppi M, Canciani M. Differentiation of bacterial and viral community-acquired pneumonia in children. Pediatr Int. 2009;51(1):91–6.
Bordley WC, Viswanathan M, King VJ, et al. Diagnosis and testing in bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2004;158(2):119–26.
Babady NE. The FilmArray® respiratory panel: an automated, broadly multiplexed molecular test for the rapid and accurate detection of respiratory pathogens. Expert Rev Mol Diagn. 2013;13(8):779–88.
Prill MM, Iwane MK, Edwards KM, et al. Human coronavirus in young children hospitalized for acute respiratory illness and asymptomatic controls. Pediatr Infect Dis J. 2012;31(3):235–40.
Talbot HK, Shepherd BE, Crowe JE Jr, et al. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J. 2009;28(8):682–7.
Lepiller Q, Barth H, Lefebvre R, et al. High incidence but low burden of coronaviruses and preferential associations between respiratory viruses. J Clin Microbiol. 2013;51(9):3039–46.
Lee J, Storch G. Characterisation of human coronavirus OC43 and uman coronavirus NL63 infections among hospitalized children < 5 years of age. Pediatr Infect Dis J. 2014;33(8):814–20.
Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J. 2008;27(12):1103–7.
Louie JK, Roy-Burman A, Guardia-Labar L, et al. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr Infect Dis J. 2009;28(4):337–9.
van Piggelen RO, van Loon AM, Krediet TG, Verboon-Maciolek MA. Human rhinovirus causes severe infection in preterm infants. Pediatr Infect Dis J. 2010;29(4):364–5.
García-García ML, Calvo C, Pozo F, Villadangos PA, Pérez-Breña P, Casas I. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(8):808–13.
Lenaerts L, De Clercq E, Naesens L. Clinical features and treatment of adenovirus infections. Rev Med Virol. 2008;18(6):357–74.
Juven T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19(4):293–8.
Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113(4):701–7.
Cevey-Macherel M, Galetto-Lacour A, Gervaix A, et al. Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr. 2009;168(12):1429–36.
Duh D, Cimerman M, Soršak K, et al. Comparison of the FilmArray respiratory panel and real-time polymerase chain reaction (RT-PCR) for detection of human adenoviruses, rhinoviruses and enteroviruses in clinical samples of paediatric patients. In: 23rd European Congress of clinical microbiology and infectious diseases, Berlin, Germany, April 27 to 30, 2013. Basel, Switzerland: European Society for Clinical Microbiology and Infectious Diseases; 2013.
Jacobs SE, Lamson DM, George K St, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013;26(1):135–62.
Hall CB, Walsh EE. Respiratory syncytial virus (Chapter 27). In: Feigin RD, Cherry JD, Demmler-Harrison GJ and Kaplan S (eds). Textbook of pediatric infectious diseases. 6th edn. Philadelphia: Saunders; 2009. pp. 2462–87.
Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children < 5 years old. J Infect Dis. 1997;175(4):807–13.
Martin ET, Fairchok MP, Kuypers J, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis. 2010;201(11):1625–32.
Vicente D, Cilla G, Montes M, Perez-Yarza EG, Perez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13(4):636–7.
Deng Y, Gu X, Zhao X, et al. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One. 2012;7:e34353. doi:10.1371/journal.pone.0034353.
Bicer S, Giray T, Çöl D, et al. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr. 2013;39:22. doi:10.1186/1824-7288-39-22.
Hall CB. Respiratory syncytial virus in young children. Lancet. 2010;375(9725):1500–2.
Aberle JH, Aberle SW, Pracher E, Hutter HP, Kundi M, Popow-Kraupp T. Single versus dual respiratory virus infections in hospitalized infants. Pediatr Infect Dis J. 2005;24(7):605–10.
Jartti T, Lee WM, Pappas T, et al. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32(2):314–20.
Jennings LC, Anderson TP, Werno AM, Beynon KA, Murdoch DR. Viral etiology of acute respiratory tract infections in children presenting to hospital: role of polymerase chain reaction and demonstration of multiple infections. Pediatr Infect Dis J. 2004;23(11):1003–7.
Wishaupt JO, Russcher A, Smeets LC, Versteegh FG, Hartwig NG. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128(5):1113–20.
Vrtovec L. Evidence based medicine. Acta Medico-Biotechnica. 2010;3(2):7–8.
Hara M, Takao S, Shimazu Y, Nishimura T. Three-year study of viral etiology and features of febrile respiratory tract infections in Japanese pediatric outpatients. Pediatr Infect Dis J. 2014;33(7):687–92.
Acknowledgments
We would like to thank Mrs. Urška Antonič and Mrs. Evelin Puklavec for translation and editing of the text.
Conflict of interest
Vojko Berce, Sibila Unuk, Darja Duh, Matjaž Homšak, and Maja Vičič (authors) declare that there are no actual or potential conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berce, V., Unuk, S., Duh, D. et al. Clinical and laboratory characteristics of viral lower respiratory tract infections in preschool children. Wien Klin Wochenschr 127 (Suppl 5), 255–262 (2015). https://doi.org/10.1007/s00508-015-0843-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-015-0843-2