Summary
Background
The aim of this post hoc analysis of data from the Austrian subpopulation of the EDGE study was the evaluation of the effectiveness and tolerability of vildagliptin as an add-on to an existing oral antidiabetic (OAD) monotherapy versus a combination therapy with two OADs without vildagliptin in patients with inadequately controlled type 2 diabetes.
Patients and methods
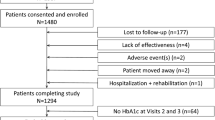
In Austria, 422 patients were included. In the framework of regular visits (at baseline, about once per quarter, and at the study end, after 12 months), adverse events (AEs), courses, and changes of therapy were recorded. In addition to the primary end point defined in the primary study, i.e., a reduction of HbA1c by > 0.3 % without hypoglycemia, weight gain ≥ 5 %, peripheral edema, or discontinuation due to gastrointestinal events, the most clinically relevant secondary end point, i.e., HbA1c reduction < 7 % without hypoglycemia or ≥ 3 % increase in body weight after 12 months was used for the analysis of the Austrian data.
Results
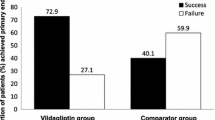
The initial HbA1c of all enrolled patients was 8.3 ± 1.4 %. The mean reduction of HbA1c was − 1.1 % in the vildagliptin cohort and − 1.0 % in the comparator cohort. In the vildagliptin cohort, 56.4 % of patients, and in the comparator cohort, 45.9 % of patients, reached the primary end point (odds ratio: 1.53, p = 0.04). In the vildagliptin cohort, 18.7 % of patients, and in the comparator cohort, 16.9 % of patients, reached the secondary end point (odds ratio: 1.13, p = 0.68). The incidence of hypoglycemic events (two in each cohort), AEs (approximately 15 % in each cohort), and serious AEs (approximately 2 % in each cohort) was comparable between the two groups.
Conclusion
In a “real-life” setting, the effectiveness of vildagliptin as second-line treatment is superior to comparator OADs with regard to a reduction in HbA1c of greater than 0.3 % from baseline without well-recognized side effects in patients with inadequately controlled type 2 diabetes (mean baseline HbA1c: 8.5 % (vildagliptin cohort) vs. 8.1 % (comparator cohort)).
Zusammenfassung
Grundlagen
Ziel dieser post hoc Analyse der Daten der österreichischen Subpopulation der EDGE Studie war es, bei Patienten mit insuffizient eingestelltem Typ 2 Diabetes Effektivität und Verträglichkeit von zwei Therapiestrategien zu vergleichen: Vildagliptin als add-on zu existierender oraler antidiabetischer Monotherapie oder eine duale orale antidiabetische Therapie ohne DPP-4 Hemmer.
Patienten und Methoden
In Österreich wurden 422 Patienten inkludiert. Im Rahmen von Routinevisiten (zu Beginn, einmal pro Quartal und zu Studienende nach 12 Monaten) wurden Nebenwirkungen, Verlauf und Änderungen der antidiabetischen Therapie dokumentiert. Der primäre Endpunkt wurde, wie in der Hauptstudie, als eine Reduktion des HbA1c-Wertes von > 0,3 % innerhalb von 12 Monaten, ohne Hypoglykämie, Gewichtszunahme ≥ 5 %, peripheren Ödemen oder Therapieabbruch wegen gastrointestinalen Nebenwirkungen definiert. Zusätzlich wurde der klinisch relevanteste sekundäre Endpunkt, eine Reduktion des HbA1c auf < 7 %, ohne Hypoglykämie oder Zunahme des Körpergewichtes ≥ 3 % für die Analyse der österreichischen Daten verwendet.
Ergebnisse
Der initiale HbA1c Wert aller eingeschlossenen Patienten war 8,3 ± 1,4 %. Die mittlere Reduktion des HbA1c betrug 1,1 % in der Vildagliptin- und 1,0 % in der Vergleichsgruppe.
In der Vildagliptingruppe erreichten 56,4 % den primären Endpunkt, in der Vergleichsgruppe 45,9 % (odds ratio 1,53, p = 0,04). Der sekundäre Endpunkt wurde von 18,7 % der Vildagliptin- und 16,9 % der Vergleichsgruppe erreicht (odds ratio 1,13, p = 0,68).
Die Inzidenz von Hypoglykämien (2 in jeder Gruppe), Nebenwirkungen (ca.15 % in jeder Gruppe) und schweren Nebenwirkungen (ca. 2 % in jeder Gruppe) war zwischen den beiden Kohorten vergleichbar.
Schlussfolgerung
In einer „real-life“ Studiensituation ist die Therapie mit Vildagliptin als zweitem oralen Antidiabetikum einer DPP-4 Hemmer freien oralen Dualtherapie bezüglich einer Reduktion des HbA1c von mehr als 0,3 % ohne klassische Nebenwirkungen überlegen (mittlerer Ausgangs-HbA1c in der Vildagliptingruppe: 8,5 %, in der Vergleichsgruppe 8,1 %).

Similar content being viewed by others
References
Clodi M, Abrahamian HE, Drexel H, Fasching P, Hoppichler F, Kautzky-Willer A, et al. Antihyperglycemic treatment guidelines for diabetes mellitus type 2. Wien Klin Wochenschr. 2012;124(Suppl):10–6.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
Ludvik B, Schernthaner G. Diabetes Care Austria 2009: registry for type 2 diabetic patients in general practitioners’ ordinations in Austria. Wien Klin Wochenschr. 2012;124(3–4):69–77.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
Treweek S, Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials. 2009;10(1):37.
Mathieu C, Barnett AH, Brath H, Conget I, de Castro JJ, Göke R, et al. Effectiveness and tolerability of second-line therapy with vildagliptin vs. other oral agents in type 2 diabetes: a real-life worldwide observational study (EDGE). Int J Clin Pract. 2013;67(10):947–56.
Davies HT. When can odds ratios mislead? BMJ. 1998;316(7136):989–91.
Blonde L, Dagogo-Jack S, Banerji MA, Pratley RE, Marcellari A, Braceras R, et al. Comparison of vildagliptin and thiazolidinedione as add-on therapy in patients inadequately controlled with metformin: results of the GALIANT trial—a primary care, type 2 diabetes study. Diabetes Obes Metab. 2009;11(10):978–86.
Bolli G, Dotta F, Colin L, Minic B, Goodman M. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab. 2009;11(6):589–95.
Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahrén B, Byiers S, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11(2):157–66.
Matthews DR, Dejager S, Ahren B, Fonseca V, Ferrannini E, Couturier A, et al. Vildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year study. Diabetes Obes Metab. 2010;12(9):780–9.
Filozof C, Gautier J-F. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diabet Med. 2010;27(3):318–26.
Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30(4):890–5.
Ware JH, Hamel MB. Pragmatic trials—guides to better patient care? N Engl J Med. 2011;364(18):1685–7.
Acknowledgments
In particular, we thank all 422 patients who were willing to share their medical data. We also thank all participating medical offices and outpatient clinics, represented by the responsible investigators Dr. Christoph Bialek, Dr. Gerald Bode, Dr. Helmut Brath, Prof. Dr. Edmund Cauza, Univ. Prof. Dr. Christoph F. Ebenbichler, Dr. Ursula Hanusch, Dr. Christian Hess, Dr. Ernst Hessenberger, Dr. Sabine Hörist-Kollmann, Dr. Peter Ilsinger, Assoc. Prof. Dr. Susanne Kaser, Dr. Marcus Peter Kufner, Univ. Prof. Dr. Anton Luger, Dr. Anita Luiskandl, Univ. Prof. Dr. Rudolf Prager, Dr. Andreas Raml, Dr. Peter Scheibner, Dr. Hans-Robert Schönherr, Dr. Günter Sokol, Dr. Felix Stockenhuber, Dr. Wolfgang Unterberger, Dr. Robert Wegmann, and Prof. Dr. Raimund Weitgasser.
Conflict of interest
Helmut Brath has received honoraria for lectures and advisory boards from all major diabetes companies, including Novartis, but has no specific conflict of interest relevant to the study; Christoph Bialek has received speaker honoraria from Novartis, Eli Lilly, MSD, Pfizer, Sanofi, Takeda; Ewald Gingl is and Michaela Ratzinger was employee of Novartis; Michael Resl has received speaker honoraria from MSD, Roche, Medtronic; Rudof Prager has received speaker honoraria from AstraZeneca, Bristol Myers Squibb, Bayer, Eli Lilly, Janssen, Cilag, Medtronic, MSD, Novartis, Novo Nordisk, Roche, Sanofi-Aventis, Takeda.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brath, H., Bialek, C., Gingl, E. et al. Efficacy and tolerability of vildagliptin-based versus comparative dual therapy in type 2 diabetes. Wien Klin Wochenschr 127, 250–255 (2015). https://doi.org/10.1007/s00508-014-0646-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-014-0646-x
Keywords
- Type 2 diabetes
- Dipeptidyl peptidase-IV inhibitor
- Oral antidiabetics
- Noninterventional trial
- Efficacy
- Tolerability