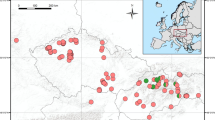
Abstract
Rubus subgen. Rubus includes common European species with highly complicated taxonomy, ongoing hybridisation and facultative apomixis. Out of approximately 750 species recognised in Europe, only 3 diploid sexual species are known, along with numerous apomictic brambles that are highly connected to polyploidy. One exception of a tetraploid taxon is R. ser. Glandulosi, which is known for prevalent sexuality. This taxon highly hybridises with tetraploid members of R. ser. Discolores and leads to the origin of many hybridogenous populations and individuals. In this study, we verify reproduction modes in different diploid, triploid and tetraploid species of subgen. Rubus, with focus on taxa putatively involved in such hybridisation by applying flow cytometric seed screen analysis. We found 100 % sexuality of diploid species, whereas triploid species had obligate unreduced embryo sac development. In contrast, tetraploid plants had varying degrees of sexuality. Additionally, we discovered that R. bifrons has the ability to undergo a reproduction mode switch as a reaction to environmental conditions. These results provide insight into reproductive modes of European brambles and shed light on their reticulate evolution and speciation.




Similar content being viewed by others
References
Adams JM (1997) Global land environments since the last interglacial. An atlas of the ice age Earth Web. http://www.esd.ornl.gov/ern/qen/nerc.html. Accessed 12 March 2011
Åkerberg GE (1939) Apomictic and sexual seed formation in Poa pratensis. Hereditas 25:359–370
Albertini E, Porceddu A, Ferranti F, Reale L, Barcaccia G, Romano B, Falcinelli M (2001) Apospory and parthenogenesis may be uncoupled in Poa pratensis: a cytological investigation. Sex Plant Reprod 14:213–217. doi:10.1007/s00497-001-0116-2
Alice LA, Campbell CS (1999) Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am J Bot 86:81–97. doi:10.2307/2656957
Amsellem L, Noyer JL, Hossaert-McKey M (2001a) Evidence for a switch in the reproductive biology of Rubus alceifolius (Rosaceae) towards apomixis, between its native range and its area of introduction. Am J Bot 88:2243–2251. doi:10.2307/3558386
Amsellem L, Chevallier MH, Hossaert-McKey M (2001b) Ploidy level of the invasive weed Rubus alceifolius Poir (Rosaceae), in its native range and in areas of introduction. Plant Syst Evol 228:171–179
Amsellem L, Pailler T, Noyer JL, Hossert-McKey M (2002) Characterisation of pseudogamous apospory in the reproductive biology of the invasive weed Rubus alceifolius (Rosaceae) in its area of introduction. Acta Bot Gallica 149:217–224
Araujo ACG, Mukhambetzhanov S, Pozzobon MT, Santana EF, Carneiro VTC (2000) Female gametophyte development in apomictic and sexual Brachiaria brizantha (Poaceae). Rev Cytol Biol Veget Le Botaniste 23:13–26
Araujo ACG, Nobrega JM, Pozzobon MT, Carneiro VTD (2005) Evidence of sexuality in induced tetraploids of Brachiaria brizantha (Poaceae). Euphytica 144:39–50. doi:10.1007/s10681-005-2842-2
Asker SE, Jerling L (1992) Apomixis in Plants. CRC Press, Boca Raton
Atkinson TC, Briffa KR, Coope GR (1987) Seasonal temperatures in Britain during the past 22,000 years, reconstructed using beetle remains. Nature 325:587–592. doi:10.1038/325587a0
Baroux C, Spillane C, Grossniklaus U (2002) Evolutionary origins of the endosperm in flowering plants. Genome Biol 30:1026
Bashaw EC, Hussey MA, Hignight KW (1992) Hybridization (n + n and 2n + n) of facultative apomictic species in the Pennisetum agamic complex. Int J Plant Sci 153:466–470. doi:10.1086/297053
Bennett MD, Leitch IJ (2011) Nuclear DNA amounts in angiosperms: tagets, trends and tomorrow. Ann Bot 107:467–590. doi:10.1093/aob/mcq258
Bicknell RA, Lambie SC, Butler RC (2003) Quantification of progeny classes, in two facultatively apotnictic accessions of Hieracium. Hereditas 138:11–20. doi:10.1034/j.1601-5223.2003.01624.x
Böcher TW (1951) Cytological and embryological studies in the amphi-apomictic Arabis holboellii complex. Det Kongelige Danske Videnskabernes Selskab 6:1–59
Cáceres ME, Matzk F, Busti A, Pupilli F, Arcioni S (2001) Apomixis and sexuality in Paspalum simplex: characterization of the mode of reproduction in segregating progenies by different methods. Sex Plant Reprod 14:201–206. doi:10.1007/s00497-001-0109-1
Campbell CS, Greene CW, Dickinson TA (1991) Reproductive biology in subfam. Maloideae (Rosaceae). Syst Bot 16:333–349. doi:10.2307/2419284
Campbell CS, Greene CW, Neubauer BF, Higgins JM (1985) Apomixis in Amelanchier laevis, shadbush (Rosaceae, Maloideae). Am J Bot 72:1397–1403. doi:10.2307/2443512
Carman JG (1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linn Soc 61:51–94. doi:10.1111/j.1095-8312.1997.tb01778.x
Carman JG, Jamison M, Elliott E, Dwivedi KK, Naumova TN (2011) Apospory appears to accelerate onset of meiosis and sexual embryo sac formation in Sorghum ovules. BMC Plant Biol 11. doi: 10.1186/1471-2229-11-9
Christen HR (1950) Untersuchungen über die Embryologie pseudogamer und sexueller Rubus-Arten. Ber Schweiz Bot Ges 60:153–198
Clark JR, Stafne ET, Hall HK, Finn CE (2007) Blackberry breeding and genetics. In: Janick J (ed) Plant breeding reviews 29. Wiley, Hoboken
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836–846
Craig DL (1960) Studies on the cytology and the breeding behaviour of Rubus canadensis L. Can J Genet Cytol 2:96–102
Czapik R (1996) Problems of apomictic reproduction in the families Compositae and Rosaceae. Folia Geobot Phytotax 31:381–387. doi:10.1007/bf02815382
Dickson EE, Arumuganathan K, Kresovich S, Doyle JJ (1992) Nuclear DNA content variation within the Rosaceae. Am J Bot 79:1081–1086
Doležel J, Binarová P, Lucretti S (1989) Analysis of nuclear DNA content in plant cells by flow-cytometry. Biol Plantarum 31:113–120
Einset J (1951) Apomixis in American polyploid blackberries. Am J Bot 38:768–772
Evans LT, Knox RB (1969) Environmental control of reproduction in Themeda australis. Aust J Bot 17:375–389
Finn CE (1996) Emasculated trailing blackberry flowers set some drupelets when not protected from cross pollination. Hort Sci 31:1035
Focke WO (1910-1914) Species Ruborum. Monographiae Generis Rubi prodromus. Bibl Bo. 72:1–118 (1910), 119–223 (1911), 83:224–498 (1914)
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051. doi:10.1126/science.220.4601.1049
Galla G, Barcaccia G, Schallau A, Molins MP, Baumlein H, Sharbel TF (2011) The cytohistological basis of apospory in Hypericum perforatum L. Sex Plant Reprod 24:47–61. doi:10.1007/s00497-010-0147-7
Gounaris EK, Sherwood RT, Gounaris I, Hamilton RH, Gustine DL (1991) Inorganic salts modify embryo sac development in sexual and aposporous Cenchrus ciliaris. Sex Plant Reprod 4:188–192
Greilhuber J (1988) `Self-tanning’—a new and important source of stoichiometric error in cytophotometric determination of nuclear DNA content in plants. Plant Syst Evol 158:87–96
Grazi F, Umaerus M, Åkerberg E (1961) Observations on the mode of reproduction and embryology of Poa pratensis. Hereditas 47:489–541
Grimanelli D, Hernandez M, Perotti E, Savidan Y (1997) Dosage effects in the endosperm of diplosporous apomictic Tripsacum (Poaceae). Sex Plant Reprod 10:279–282. doi:10.1007/s004970050098
Gustafsson A (1930) Kastrierungen und Pseudogamie bei Rubus. Bot not 83:477–494
Gustafsson A (1942) The origin and properties of the European blackberry flora. Hereditas 28:249–277
Gustafsson A (1943) The genesis of the European blackberry flora. Acta Universitet Lundskrift 39:1–200
Hamilton WD (1980) Sex versus non-sex versus parasite. Oikos 35:282–290. doi:10.2307/3544435
Harlan JR, de Wet JMJ (1975) On Ö. Winge and a prayer: the origins of polyploidy. Bot Rev 41:361–390
Haskell G (1960) Role of the male parent in crosses involving apomictic Rubus species. Heredity 14:101–113
Hintze J (2012) NCSS 8. NCSS, LLC. Kaysville, Utah. www.ncss.com
Holm S, Ghatnekar L, Bengtsson BO (1997) Selfing and outcrossing but no apomixis in two natural populations of diploid Potentilla argentea. J Evol Biol 10:343–352. doi:10.1007/s000360050028
Hörandl E (2006) The complex causality of geographical parthenogenesis. New Phytol 171:525–538. doi:10.1111/j.1469-8137.2006.01769.x
Hörandl E, Temsch EM (2009) Introgression of apomixis into sexual species is inhibited by mentor effects and ploidy barriers in the Ranunculus auricomus complex. Ann Bot 104:81–89. doi:10.1093/aob/mcp093
Houliston GJ, Chapman HM, Bicknell RA (2006) The influence of genotype and environment on the fecundity and facultative expression of apomixis in Hieracium pilosella. Folia Geobot 41:165–181. doi:10.1007/bf02806477
Jedrzejczyk I, Sliwinska E (2010) Leaves and seeds as materials for flow cytometric estimation of the genome size of 11 Rosaceae woody species containing DNA-staining inhibitors. J Bot. doi: 10.1155/2010/930895
Jennings DL, Craig DL, Topham PB (1967) The role of the male parent in the reproduction of Rubus. Heredity 22:43–55
Kaushal P, Malaviya DR, Roy AK, Pathak S, Agrawal A, Khare A, Siddiqui SA (2008) Reproductive pathways of seed development in apomictic guinea grass (Panicum maximum Jacq.) reveal uncoupling of apomixis components. Euphytica 164:81–92. doi:10.1007/s10681-008-9650-4
Kearney M (2005) Hybridization, glaciation and geographical parthenogenesis. Trends Ecol Evol 20:495–502. doi:10.1016/j.tree.2005.06.005
Kerr EA (1954) Seed development in blackberries. Can J Bot 32:654–672
Kimber G, Riley R (1963) Haploid angiosperms. Bot Rev 29:480–531
Knox RB (1967) Apomixis: seasonal and population differences in a grass. Science 157:325–326
Kollmann J, Steinger T, Roy BA (2000) Evidence of sexuality in European Rubus (Rosaceae) species based on AFLP and allozyme analysis. Am J Bot 87:1592–1598. doi:10.2307/2656735
Koltunow AM (1993) Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5:1425–1437. doi:10.2307/3869793
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annu Rev Plant Biol 54:547–574. doi:10.1146/annurev.arplant.54.110901.160842
Koltunow AM, Johnson SD, Bicknell RA (1998) Sexual and apomictic development in Hieracium. Sex Plant Reprod 11:213–230. doi:10.1007/s004970050144
Kondrashov AS (1988) Deleterious mutations and the evolution of sexual reproduction. Nature 336:435–440. doi:10.1038/336435a0
Krahulcová A, Rotreklová O (2010) Use of flow cytometry in research on apomictic plants. Preslia 82:23–39
Krahulcová A, Trávníček B, Šarhanová P (2013) Karyological variation in the genus Rubus, subgenus Rubus (brambles, Rosaceae): new data from the Czech Republic and synthesis of the current knowledge from Europe. Preslia 85 (in press)
Krahulec F, Krahulcová A, Rosenbaumová R, Plačková I (2011) Production of polyhaploids by facultatively apomictic Pilosella can result in the formation of new genotypes via genome doubling. Preslia 83:471–490
Krahulec F, Krahulcová A, Fehrer J, Bräutigam S, Schuhwerk F (2008) The structure of the agamic complex of Hieracium subgen. Pilosella in the Šumava Mts and its comparison with other regions in Central Europe. Preslia 80:1–26
Kurtto A, Weber HE, Lampinen R, Sennikov AN (eds) (2010) Atlas Florae Europaeae. Distribution of vascular plants in Europe. 15. Rosaceae (Rubus). The Committee for Mapping the Flora of Europe and Societas Biologica Fennica Vanamo, Helsinki
Levin DA (1975) Minority cytotype exclusion in local plant populations. Taxon 24:35–43
Lidforss B (1914) Resumé seiner Arbeiten uber Rubus. Z. Induk. Abstamm. u. Vereb. 12:1–13
Lihová J, Mártonfi P, Mártonfiová L (2000) Experimental study on reproduction of Hypericum × desetangsii nothosubsp. carinthiacum (A. Fröhl.) N. Robson (Hypericaceae). Caryologia 53:127–132
Lysák MA, Doležel J (1998) Estimation of nuclear DNA content in Sesleria (Poaceae). Caryologia 51:123–132
Mallet J (2007) Hybrid speciation. Nature 446:279–283. doi:10.1038/nature05706
Matzk F, Meister A, Brutovska R, Schubert I (2001) Reconstruction of reproductive diversity in Hypericum perforatum L. opens novel strategies to manage apomixis. Plant J 26:275–282. doi:10.1046/j.1365-313X.2001.01026.x
Matzk F, Meister A, Schubert I (2000) An efficient screen for reproductive pathways using mature seeds of monocots and dicots. Plant J 21:97–108. doi:10.1046/j.1365-313x.2000.00647.x
Matzk F, Prodanovic S, Baumlein H, Schubert I (2005) The inheritance of apomixis in Poa pratensis confirms a five locus model with differences in gene expressivity and penetrance. Plant Cell 17:13–24. doi:10.1105/tpc.104.027359
Meng R, Finn C (2002) Determining ploidy level and nuclear DNA content in Rubus by flow cytometry. J Am Soc Hort Sci 127(5):767–775
Mogie M (1992) The evolution of asexual reproduction in plants. Chapman and Hall Press, New York
Naumova TN, Willemse MTM (1995) Ultrastructural characterization of apospory in Panicum maximum. Sex Plant Reprod 8:197–204
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angiosperms. Springer, New York, pp 475–518
Norstog KJ (1957) Polyembryony in Hierochloe odorata (L.) Beauv. Ohio J Sci 57:315–320
Nybom H (1987) Pollen limited seed set in pseudogamous blackberries (Rubus L. subgen. Rubus). Oecologia 72:562–568. doi:10.1007/bf00378983
Nybom H (1988) Apomixis versus sexuality in blackberries (Rubus subgen. Rubus, Rosaceae). Plant Syst Evol 160:207–218. doi:10.1007/bf00936048
Nybom H (1995) Evaluation of interspecific crossing experiments in facultatively apomictic blackberries (Rubus subgen. Rubus) using DNA fingerprinting. Hereditas 122:57–65. doi:10.1111/j.1601-5223.1995.00057.x
Ozias-Akins P, van Dijk PJ (2007) Mendelian genetics of apomixis in plants. Annu Rev Genet 41:509–537. doi:10.1146/annurev.genet.40.110405.090511
Paule J, Sharbel TF, Dobeš C (2011) Apomictic and sexual lineages of the Potentilla argentea L. group (Rosaceae): cytotype and molecular genetic differentiation. Taxon 60:721–732
Pfosser M, Amon A, Lelley T, Heberlebors E (1995) Evaluation of sensitivity of flow-cytometry in detecting aneuploidy in wheat using disomic and ditelosomic wheat-rye addition lines. Cytometry 21:387–393. doi:10.1002/cyto.990210412
Pratt C, Einset J (1955) Development of the embryo sac in some American blackberries. Am J Bot 42:637–645
Richards AJ (1986) Plant breeding systems. George Allen and Unwin, London
Robertson A, Rich TCG, Allen AM, Houston L, Roberts C, Bridle JR, Harris SA, Hiscock SJ (2010) Hybridization and polyploidy as drivers of continuing evolution and speciation in Sorbus. Mol Ecol 19:1675–1690. doi:10.1111/j.1365-294X.2010.04585.x
Rutishauser A (1954) Die Entwicklungserregug des Endosperms bei pseudogamen Ranunculus-Arten. Mitt D Naturforsch Ges Schaffhausen 25:1–45
Ryde U (2011) Arguments for a narrow species concept in Rubus sect. Corylifolii. Nord J Bot 29:708–721. doi:10.1111/j.1756-1051.2011.01203.x
Siena LA, Sartor ME, Espinoza F, Quarin CL, Ortiz JPA (2008) Genetic and embryological evidences of apomixis at the diploid level in Paspalum rufum support recurrent auto-polyploidization in the species. Sex Plant Reprod 21:205–215. doi:10.1007/s00497-008-0080-1
Sims LE, Price HJ (1985) Nuclear DNA content variation in Helianthus (Asteraceae). Am J Bot 72:1213–1219
Sliwinska E, Zielinska E, Jedrzejczyk I (2005) Are seeds suitable for flow cytometric estimation of plant genome size? Cytom Part A 64A:72–79
Snyder LA (1957) Apomixis in Paspalum secans. Am J Bot 44:318–324
Talent N (2009) Evolution of gametophytic apomixis in flowering plants: an alternative model from Maloid Rosaceae. Theor Biosci 128:121–138. doi:10.1007/s12064-009-0061-4
Talent N, Dickinson TA (2007a) Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. New Phytol 173:231–249. doi:10.1111/j.1469-8137.2006.01918.x
Talent N, Dickinson TA (2007b) The potential for ploidy level increases and decreases in Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae). Can J Bot 85:570–584. doi:10.1139/b07-028
Tate JA, Joshi P, Soltis KA, Soltis PS, Soltis DE (2009) On the road to diploidization? Homoeolog loss in independently formed populations of the allopolyploid Tragopogon miscellus (Asteraceae). BMC Plant Biol 9. doi:10.1186/1471-2229-9-80
Tesařová (2012) Analysis of the genome size in selected Rubus species using flow-cytometry. Bachelor thesis, Palacky University, Olomouc
Thomas PT (1940) Reproductive versatility in Rubus. II. The chromosomes and development. J Genet 40:119–128
Tucker MR, Koltunow AMG (2009) Sexual and asexual (apomictic) seed development in flowering plants: molecular, morphological and evolutionary relationships. Funct Plant Biol 36:490–504. doi:10.1071/fp09078
van Dijk PJ (2003) Ecological and evolutionary opportunities of apomixis: insights from Taraxacum and Chondrilla. Philos T Roy Soc B 358:1113–1120. doi:10.1098/rstb.2003.1302
Vinkenoog R, Scott RJ (2001) Autonomous endosperm development in flowering plants: how to overcome the imprinting problem. Sex Plant Reprod 14:189–194. doi:10.1007/s00497-001-0106-4
Vít P, Lepší M, Lepší P (2012) There is no diploid apomict among Czech Sorbus species: a biosystematics revision of S. eximia and discovery of S. barrandienica. Preslia 84:71–96
Voigt ML, Melzer M, Rutten T, Mitchell-Olds T, Sharbel TF (2007) Gametogenesis in the apomictic Boechera holboellii complex: the male perspective. In: Hörandl E, Grossniklaus U, van Dijk PJ, Sharbel TF (eds) Apomixis: evolution, mechanisms and perspectives. ARG Gantner Verlag, Ruggell, pp 235–258
Weber HE (1995) 4. Rubus. In: Hegi G (ed) Illustrierte Flora von Mitteleuropa, edn 3, vol 4/2A. Blackwell, Berlin, pp 284–595
Weber HE (1996) Former and modern taxonomic treatment of the apomictic Rubus complex. Folia Geobot Phytotax 31:373–380. doi:10.1007/bf02815381
Werlemark G, Nybom H (2003) Pollen donor impact on progenies of pseudogamous blackberries (Rubus subgen. Rubus). Euphytica 133:71–80. doi:10.1023/a:1025674128000
West SA, Lively CM, Read AF (1999) A pluralist approach to sex and recombination. J Evol Biol 12:1003–1012
Whitton J, Sears CJ, Baack EJ, Otto SP (2008) The dynamic nature of apomixis in the angiosperms. Int J Plant Sci 169:169–182. doi:10.1086/523369
Acknowledgments
We thank all colleagues who helped in the fieldwork with finding the localities of bramble species, namely M. Lepší (České Budějovice), P. Lepší (Český Krumlov) and V. Žíla (Strakonice). We also thank Tim Sharbel for comments regarding the manuscript. M. Tesařová and K. Truhlářová (Palacký University, Olomouc) were students who performed part of the confirmatory flow cytometric analyses. This study was supported by the Grant Agency of the Czech Republic (grants no. 206/07/0706 and 206/08/0890) and by the internal grant from Palacký University (PrF 2012/001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Scott Russell.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Šarhanová, P., Vašut, R.J., Dančák, M. et al. New insights into the variability of reproduction modes in European populations of Rubus subgen. Rubus: how sexual are polyploid brambles?. Sex Plant Reprod 25, 319–335 (2012). https://doi.org/10.1007/s00497-012-0200-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-012-0200-9