Abstract
Key message
Initial stomatal conductance at onset of illumination and lag times before stomata start to open in response to light are decisive for the whole course of photosynthetic induction. Neglecting these effects can reverse results when comparing induction parameters within and between species.
Abstract
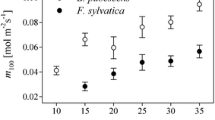
We analyzed stomatal behaviors, the role of initial stomatal conductance (at onset of light, g ini), and contemporaneous effects on two types of photosynthetic induction, namely “overall” (stomatal and mesophyll effects included) and “biochemical induction” (mainly RuBisCO activity). Studies were performed in sun leaves of four species (Alnus glutinosa (L.) Gaertn., Betula pubescens Ehrh., Fagus sylvatica L., and Helianthus annuus L.) differing in shade-tolerance: A species-specific g ini-threshold, namely g ini(crit), was found, being decisive for time courses of induction. When g ini was above g ini(crit), induction half-times (t 50%) were reached within 5 min, almost completely independent of stomatal limitations, and both induction types did not differ between species. Large variations in t 50% (4–36 min) were observed when g ini < g ini(crit). These correlated linearly with lag times before onset of stomatal opening in response to light (t lag). t lag is generally the longer the lower g ini. Total overall and total biochemical induction correlated highly significantly with velocities of stomatal opening. Different induction times between species occurred as a result of stomatal behavior rather than photosynthetic parameters. Any induction process is initially more limited by the biochemical component. When g ini < g ini(crit), this biochemical limitation shifts towards stomata-dominated limitation at a lower induction state. Consequentially, stomata limit the induction process over most of the induction course. When g ini > g ini(crit) and stomata open fast, then induction is (almost) completely dominated by biochemical limitations and proceeds much faster. This study confirms the very important role of g ini(crit) in photosynthetic induction irrespective of species and discusses implications for modeling.








Similar content being viewed by others
Abbreviations
- Γ*:
-
Light-independent CO2 compensation point
- ΔA120 :
-
Increase of photosynthesis after 120 s of illumination (A–R D)
- Δw:
-
Leaf/air vapor concentration difference
- A :
-
Net photosynthesis
- A ind :
-
Overall photosynthetic induction
- A max :
-
Maximum A at full photosynthetic induction
- B ind :
-
Biochemical photosynthetic induction
- C a :
-
Ambient CO2 concentration
- C i :
-
Intercellular CO2 concentration
- g :
-
Stomatal conductance to H2O
- g c :
-
Cuticular conductance to H2O
- g ini :
-
g before illumination
- g l :
-
Leaf conductance to H2O
- g m :
-
Mesophyll conductance to CO2
- g max :
-
g at full induction
- IS120 :
-
Induction state after 120 s of illumination
- ISA=B :
-
Induction state at which A ind equals B ind
- m 100 :
-
Initial slope of the A/C i relationship at full induction
- PPFD:
-
Photosynthetic photon flux density
- R D :
-
Leaf respiration in darkness
- R I :
-
Leaf respiration in light
- RuBisCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- RuBP:
-
Ribulose-1,5-bisphosphate
- t :
-
Time
- t 50%A and t 90%A :
-
Time to reach 50% and 90% of A ind
- t 50%B and t 90%B :
-
Time to reach 50% and 90% of B ind
- t 90%g :
-
Time to reach 90% of g max
- t A=B :
-
Time to reach ISA=B
- t lag :
-
Lag time in stomatal response after beginning of illumination
References
Allen MT, Pearcy RW (2000) Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia 122:470–478. doi:10.1007/s004420050968
Assmann SM (1999) The cellular basis of guard cell sensing of rising CO2. Plant Cell Environ 22:629–637. doi:10.1046/j.1365-3040.1999.00408.x
Auchincloss L, Easlon HM, Levine D et al (2014) Pre-dawn stomatal opening does not substantially enhance early-morning photosynthesis in Helianthus annuus. Plant Cell Environ 37:1364–1370. doi:10.1111/pce.12241
Azoulay-Shemer T, Palomares A, Bagheri A et al (2015) Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2- and ABA-induced stomatal closing. Plant J 83:567–581. doi:10.1111/tpj.12916
Ball JT, Woodrow IE, Berry JA (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: Biggins J (ed.) Progress in Photosynthesis Research. Martinus Nijhoff Publishers, Dordrecht, pp 221–224
Bazzaz FA (1979) The physiological ecology of plant succession. Ann Rev Ecol Syst 10:351–371
Bernacchi CJ, Singsaas EL, Pimentel C et al (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259. doi:10.1046/j.1365-3040.2001.00668.x
Beyschlag W, Pfanz H, Ryel R (1992) Stomatal patchiness in Mediterranean evergreen sclerophylls. Phenomenology and consequences for the interpretation of the midday depression in photosynthesis and transpiration. Planta 187:546–553
Bradshaw RHW, Wolf A, Møller PF (2005) Long-term succession in a Danish temperate deciduous forest. Ecography (Cop) 28:157–164. doi:10.1111/j.0906-7590.2005.03980.x
Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Estimates from gas-exchange measurements on spinach. Planta 165:397–406
Chazdon RL, Pearcy RW (1986) Photosynthetic responses to light variation in rainforest species. I. Induction under constant and fluctuating light conditions. Oecologia 69:517–523
Damour G, Simonneau T, Cochard H, Urban L (2010) An overview of models of stomatal conductance at the leaf level. Plant Cell Environ 33:1419–1438. doi:10.1111/j.1365-3040.2010.02181.x
Douthe C, Dreyer E, Brendel O, Warren CR (2012) Is mesophyll conductance to CO2 in leaves of three Eucalyptus species sensitive to short-term changes of irradiance under ambient as well as low O2? Funct Plant Biol 39:435–448. doi:10.1071/FP11190
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153. doi:10.1111/j.1365-3040.2004.01140.x
Farquhar GD, Sharkey TD (1982) Stomatal Conductance and Photosynthesis. Annu Rev. Plant Physiol 33:317–345. doi:10.1146/annurev.pp.33.060182.001533
Farquhar GD, Wong SC (1984) An Empirical Model of Stomatal Conductance. Funct Plant Biol 11:191–210. doi:10.1071/PP9840191
Flexas J, Barbour MM, Brendel O et al (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193–194:70–84. doi:10.1016/j.plantsci.2012.05.009
Fujita T, Noguchi K, Terashima I (2013) Apoplastic mesophyll signals induce rapid stomatal responses to CO2 in Commelina communis. New Phytol 199:395–406. doi:10.1111/nph.12261
Hoad SP, Grace J, Jeffree CE (1997) Humidity response of cuticular conductance of beech (Fagus sylvatica L.) leaf discs maintained at high relative water content. J Exp Bot 48:1969–1975. doi:10.1093/jxb/48.11.1969
Holišová P, Zitová M, Klem K, Urban O (2012) Effect of elevated carbon dioxide concentration on carbon assimilation under fluctuating light. J Environ Qual 41:1931–1938. doi:10.2134/jeq2012.0113
Jarvis PG (1976) The interpretation of the variations in leaf water potential and stomatal conductance found in canopies in the field. Philos Trans R Soc London B 273:593–610.
Kaiser H, Kappen L (2001) Stomatal oscillations at small apertures: indications for a fundamental insufficiency of stomatal feedback-control inherent in the stomatal turgor mechanism. J Exp Bot 52:1303–1313. doi:10.1093/jexbot/52.359.1303
Kirschbaum MUF, Pearcy RW (1988a) Gas exchange analysis of the fast phase of photosynthetic induction in Alocasia macrorrhiza. Plant Physiol 87:818–821. doi:10.1104/pp.87.4.818
Kirschbaum MUF, Pearcy RW (1988b) Gas exchange analysis of the relative importance of stomatal and biochemical factors in photosynthetic induction in Alocasia macrorrhiza. Plant Physiol 86:782–785. doi:10.1104/pp.87.4.818
Kirschbaum MUF, Gross LJ, Pearcy RW (1988) Observed and modelled stomatal responses to dynamic light environments in the shade plant Alocasia macrorrhiza. Plant Cell Environ 11:111–121
Kirschbaum MUF, Küppers M, Schneider H et al (1998) Modelling photosynthesis in fluctuating light with inclusion of stomatal conductance, biochemical activation and pools of key photosynthetic intermediates. Planta 204:16–26. doi:10.1007/s004250050225
Kirschbaum MUF, Oja V, Laisk A (2005) The quantum yield of CO2 fixation is reduced for several minutes after prior exposure to darkness. Exploration of the underlying causes. Plant Biol 7:58–66. doi:10.1055/s-2004-830476
Kobza J, Edwards GE (1987) The photosynthetic induction response in wheat leaves: net CO2 uptake, enzyme activation, and leaf metabolites. Planta 171:549–559. doi:10.1007/BF00392305
Koike T (1988) Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol 3:77–87
Košvancová M, Urban O, Šprtová M et al (2009) Photosynthetic induction in broadleaved Fagus sylvatica and coniferous Picea abies cultivated under ambient and elevated CO2 concentrations. Plant Sci 177:123–130. doi:10.1016/j.plantsci.2009.04.005
Küppers M (1984) Carbon relations and competition between woody species in a Central European Hedgerow. I. Photosynthetic characteristics. Oecologia (Berl) 64:332–343.
Küppers M, Schneider H (1993) Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: effects of fleck length and leaf temperature in leaves grown in deep and partial shade. Trees 7:160–168. doi:10.1007/BF00199617
Küppers M, Schulze ED (1985) An empirical model of net photosynthesis and leaf conductance for the simulation of diurnal courses of CO2 and H2O exchange. Aust J Plant Physiol 12:513–526
Küppers M, Wheeler AM, Küppers BIL, et al (1986) Carbon fixation in eucalypts in the field. Analysis of diurnal variations in photosynthetic capacity. Oecologia (Berl) 70:273–282.
Küppers M, Swan AG, Tompkins D et al (1987) A field portable system for the measurement of gas exchange of leaves under natural and controlled conditions: examples with field-grown Eucalyptus pauciflora Sieb. ex Spreng. ssp. pauciflora, E. behriana F. Muell. and Pinus radiata R. Don. Plant Cell Environ 10:425–435. doi:10.1111/1365-3040.ep11603690
Küppers BIL, Küppers M, Schulze ED (1988) Soil drying and its effect on leaf conductance and CO2 assimilation of Vigna unguiculata (L.) Walp. I. The response to climatic factors and to the rate of soil drying in young plants. Oecologia 75:99–104
Küppers M, Timm HC, Orth F et al (1996) Effects of light environment and successional status on lightfleck use by understory trees of temperate and tropical forests. Tree Physiol 16:69–80
Küppers M, Heiland I, Schneider H, Neugebauer PJ (1999) Light-flecks cause non-uniform stomatal opening—studies with special emphasis on Fagus sylvatica L. Trees 14:130. doi:10.1007/s004680050218
Lawson T, Simkin AJ, Kelly G, Granot D (2014) Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol 203:1064–1081. doi:10.1111/nph.12945
Lehman RH, Rice EL (1972) Effect of deficiencies of nitrogen, potassium and sulfur on chlorogenic acids and scopolin in sunflower. Amer Midl Nat 87:71–80.
Mansfield TA, Atkinson CJ (1990) Stomatal Behaviour in Water Stressed Plants. In: Alscher RG, Cumming JR (eds.) Stress responses in plants: adaptation and acclimation mechanisms. Wiley-Liss, New York, pp 241–264
Mott KA, Peak D (2007) Stomatal patchiness and task-performing networks. Ann Bot 99:219–226. doi:10.1093/aob/mcl234
Mott KA, Woodrow IE (1993) Effects of O2 and CO2 on Nonsteady-State Photosynthesis. Plant Physiol 102:859–866
Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31:1299–1306. doi:10.1111/j.1365-3040.2008.01845.x
Mott KA, Berg DG, Hunt SM, Peak D (2014) Is the signal from the mesophyll to the guard cells a vapour-phase ion? Plant Cell Environ 37:1184–1191. doi:10.1111/pce.12226
Naumburg E, Ellsworth DS (2000) Photosynthetic sunfleck utilization potential of understory saplings growing under elevated CO2 in FACE. Oecologia 122:163–174. doi:10.1007/PL00008844
Naumburg E, Ellsworth DS (2002) Short-term light and leaf photosynthetic dynamics affect estimates of daily understory photosynthesis in four tree species. Tree Physiol 22:393–401
Ögren E, Sundin U (1996) Photosynthetic responses to variable light†¯: a comparison of species from contrasting habitats. Oecologia 106:18–27
Pearcy RW, Chazdon RL, Gross LI, Mott KA (1994) Photosynthetic utilization of sunflecks: a temporally patchy resource on a time scale of seconds to minutes. In: Caldwell MM, Pearcy RW (eds.) Exploitation of environmental heterogeneity by plants. Academic Press, San Diego, pp 175–208
Pearcy RW, Gross LJ, He D (1997) An improved dynamic model of photosynthesis for estimation of carbon gain in sunfleck light regimes. Plant Cell Environ 20:411–424
Portis AR (2003) Rubisco activase—Rubisco’s catalytic chaperone. Photosynth Res 75:11–27
Pospíšilová J, Šantrůček J (1994) Stomatal patchiness. Biol Plant 36:481–510. doi:10.1007/BF02921169
Radin JW, Lu Z, Percy RG, Zeiger E (1994) Genetic variability for stomatal conductance in Pima cotton and its relation to improvements of heat adaptation. Proc Natl Acad Sci USA 91:7217–7221. doi:10.1073/pnas.91.15.7217
Raschke K (1979) Movements of stomata. In: Haupt W, Feinleib ME (eds.) Encyclopedia of plant physiology NS, vol VII. Springer, Berlin, pp 383–441
Resco de Dios V, Gessler A, Ferrio JP et al (2016) Circadian rhythms have significant effects on leaf-to-canopy scale gas exchange under field conditions. Gigascience 5:43. doi:10.1186/s13742-016-0149-y
Sassenrath-Cole GF, Pearcy RW (1992) The role of Ribulose-1,5-bisphosphate regeneration in the induction requirement of photosynthetic co(2) exchange under transient light conditions. Plant Physiol 99:227–234. doi:10.1104/pp.99.1.227
Seemann JR, Kirschbaum MUF, Sharkey TD, Pearcy RW (1988) Regulation of Ribulose-1,5-bisphosphate carboxylase activity in Alocasia macrorrhiza in response to step changes in irradiance. Plant Physiol 88:148–152. doi:10.1104/pp.88.1.148
Stegemann J, Timm HC, Küppers M (1999) Simulation of photosynthetic plasticity in response to highly fluctuating light: an empirical model integrating dynamic photosynthetic induction and capacity. Trees 14:145–160. doi:10.1007/s004680050219
Terashima I, Wong SC, Osmond CB, Farquhar GD (1988) Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant Cell Physiol 29:385–394
Timm HC, Küppers M, Stegemann J (2004) Non-destructive analysis of architectural expansion and assimilate allocation in different tropical tree saplings: consequences of using steady-state and dynamic photosynthesis models. Ecotropica 10:101–121
Tinoco-Ojanguren C, Pearcy RW (1993) Stomatal dynamics and its importance to carbon gain in two rainforest Piper species—I. VPD effects on the transient stomatal response to lightflecks. Oecologia 94:388–394. doi:10.1007/BF00317114
Tomimatsu H, Tang Y (2012) Elevated CO2 differentially affects photosynthetic induction response in two Populus species with different stomatal behavior. Oecologia 169:869–878. doi:10.1007/s00442-012-2256-5
Urban O, Košvancová M, Marek MV, Lichtenthaler HK (2007) Induction of photosynthesis and importance of limitations during the induction phase in sun and shade leaves of five ecologically contrasting tree species from the temperate zone. Tree Physiol 27:1207–1215. doi:10.1093/treephys/27.8.1207
Urban O, Sprtová M, Košvancová M et al (2008) Comparison of photosynthetic induction and transient limitations during the induction phase in young and mature leaves from three poplar clones. Tree Physiol 28:1189–1197
Valladares F, Allen MT, Pearcy RW (1997) Photosynthetic responses to dynamic light under field conditions in six tropical rainforest shrubs occuring along a light gradient. Oecologia 111:505–514. doi:10.1007/s004420050264
Vialet-Chabrand S, Dreyer E, Brendel O (2013) Performance of a new dynamic model for predicting diurnal time courses of stomatal conductance at the leaf level. Plant Cell Environ 36:1529–1546. doi:10.1111/pce.12086
Vico G, Manzoni S, Palmroth S, Katul G (2011) Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes. New Phytol 192:640–652. doi:10.1111/j.1469-8137.2011.03847.x
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387. doi:10.1007/BF00384257
Way DA, Pearcy RW (2012) Sunflecks in trees and forests: From photosynthetic physiology to global change biology. Tree Physiol 32:1066–1081. doi:10.1093/treephys/tps064
Woodrow IE, Mott KA (1989) Rate limitation of non-steady-state photosynthesis by Ribulose-1,5-bisphosphate carboxylase in Spinach. Aust J Plant Physiol 16:487. doi:10.1071/PP9890487
Zhang J, Davies WJ (1990) Changes in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant Cell Environ 13:277–285
Zhang Q, Chen JW, Li BG, Cao KF (2009) Epiphytes and hemiepiphytes have slower photosynthetic response to lightflecks than terrestrial plants: evidence from ferns and figs. J Trop Ecol 25:465. doi:10.1017/S026646740900618X
Zhang Q, Chen YJ, Song LY et al (2012) Utilization of lightflecks by seedlings of five dominant tree species of different subtropical forest successional stages under low-light growth conditions. Tree Physiol 32:545–553. doi:10.1093/treephys/tps043
Acknowledgements
We thank Miko Kirschbaum (Palmerston North, New Zealand) and unknown reviewers for very helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Gessler.
Rights and permissions
About this article
Cite this article
Wachendorf, M., Küppers, M. The effect of initial stomatal opening on the dynamics of biochemical and overall photosynthetic induction. Trees 31, 981–995 (2017). https://doi.org/10.1007/s00468-017-1522-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-017-1522-x