Abstract
Key message
We provide new embryological information regarding the Fagaceae family. Abortive ovules may be a result of mate choice and be necessary to maximize reproductive success in Chinese chinquapin ( Castanea henryi ).
Abstract
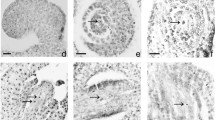
In contrast to most angiosperms, in which fertilization occurs 1–2 days after pollination, fertilization in Fagales is delayed from 4 days to more than 1 year. However, the phenomenon of delayed fertilization in Castanea Miller species (Fagaceae), which are the economically important species of Fagaceae and widely distributed or cultured in many countries of the world, has been consistently neglected, raising questions regarding what the delayed fertilization process is and why fertilization is delayed. To answer these questions, we systematically investigated the micro- and megasporogenesis in addition to male and female gametogenesis in Castanea henryi (Castanea Miller species). Our results show that the ovules primordia are immature at the time of pollination and require 6 weeks to become fully developed. During this 6-week period, 32.30 % of ovules abort because of the inability to form an embryo sac (ES). Approximately 15.79 % of ovules abort because of abnormal development of ES. From 7 to 8 weeks after pollination in which double fertilization occurs, most mature ovules are also aborted because of cell degeneration in gametophytes. Only one ovule can develop into a ripe seed. Thus, the delayed fertilization in Castanea henryi may be necessary to increase the time for mate choice and selective fertilization. A certain number of ovule abortions may be the result of delayed fertilization to maximize reproductive success.







Similar content being viewed by others
References
Andrea L, Lrina K, Kanok-orn S, Thoma D (2010) Sporophytic control of pollen tube growth and guidance in maize. J Exp Bot 61:673–682
Bertin RI, Newman CM (1993) Dichogamy in angiosperms. Bot Rev 59(2):112–152
Boavida LC, Varela MC, Feijo JA (1999) Sexual reproduction in the cork oak (Quercus suber L.). I. The programic phase. Sex Plant Reprod 11:347–353
Botta R, Vergano G, Vallania G, Me G, Vallania R (1995) Floral biology and embryo development in Chestnut (Castanea sativa Mill.). HortScience 30:1283–1286
Dahl AE, Fredrikson M (1996) The timetable for development of maternal tissues sets the stage for male genomic selection in Betula pendula (Betulaceae). Am J Bot 83(7):895–902
Dane F, Lang P, Huang H, Fu Y (2003) Intercontinental genetic divergence of Castanea species in eastern Asia and eastern North America. Heredity 91(3):314–321
Davis GL (1966) Systematic embryology of the angiosperm. Wiley, New York, pp 283–505
Deng M, Zhou ZK, Chen YQ, Sun WB (2008) Systematic significance of the development and anatomy of flowers and fruit of Quercus schottkyana (Subgenus cyclobalanopsis: Fagaceae). Int J Plant Sci 169(9):1261–1277
Distefano G, Gentile A, Herrero M (2011) Pollen-pistil interactions and early fruiting in parthenocarpic citrus. Ann Bot 108:499–509
Gomez JM, Zamora R (2003) Factors affecting intrafruit pattern of ovule abortion and seed production in Hormathophylla spinosa (Cruciferae). Plant Syst Evol 239:215–229
Herrero M (2003) Male and female synchrony and the regulation of mating in flowering plant. Phil Trans R Soc Lond B 358:1019–1024
Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T (2001) Pollen tube attraction by the synergid cell. Science 293:1480–1483
Higashiyama T, Kuroiwa H, Kuroiwa T (2003) Pollen-tube guidance: beacons from the female gametophyte. Curr Opin Plant Biol 6(1):6–41
Hufford KM, Hamrick JL (2003) Viability selection at three early life stages of the tropical tree, Platypodium elegans (Fabaceae, Papilionoideae). Evolution 57:518–526
Kalisz S, Randle A, Chaiffetz D, Faigeles M, Butera A, Craig B (2011) Dichogamy correlates with outcrossing rate and defines the selfing syndrome in the mixed-mating genus Collinsia. Ann Bot 109(3):571–582
Kapil RN, Sethi SB (1963) Development of male and female gametophytes in Camellia sinensis (L.) O. Kuntze. Proc Natl Inst Sci India B 29:574–579
Lang P, Dane F, Kubisiak TL, Huang HW (2007) Molecular evidence for an Asian origin and a unique westward migration of species in the genus Castanea via Europe to North America. Mol Phylogenet Evol 43:49–59
Leshem Y, Johnson C, Sundaresan V (2013) Pollen tube entry into the synergid cell of Arabidopsis is observed at a site distinct from the fifiform apparatus. Plant Reprod 26(2):93–99
Li HJ, Xue Y, Jia DJ, Wang T, Shi DQ, Liu J, Cui F, Xie F, Xie Q, Ye D, Yang WC (2011) POD1 regulates pollen tube guidance in response to micropylar female signaling and acts in early embryo patterning in Arabidopsis. Plant Cell 23:3288–3302
Liu JF, Cheng YP, Yan K, Liu Q, Wang ZW (2012) The relationship between reproductive growth and blank fruit formation in Corylus heterophylla Fisch. Sci Hortic 136:128–134
Liu JF, Zhang HD, Cheng YQ, Kafkas S, Güney M (2014) Pistillate flower development and pollen tube growth mode during the delayed fertilization stage in Corylus heterophylla Fisch. Plant Reprod 27(3):145–152
Lo HC, Huang TM (2005) Microsporogenesis in Cyclobalanopsis glauca. J Exp For Nat Taiwan Univ 19(1):55–68
Losada JM, Herrero M (2014) Glycoprotein composition along the pistil of Malus × domestica and the modulation of pollen tube growth. BMC Plant Biol 14:1–14
Ma GH, Bunn E, Zhang JF, Wu GJ (2006) Evidence of Dichogamy in Santalum album L. J Integr Plant Biol 48(3):300–306
Mahs A, Steinhorst L, Han JP, Shen LK, Wang Y, Kudla J (2013) The calcineurin B-like Ca2+ sensors CBL1 and CBL9 function in pollen germination and pollen tube growth in Arabidopsis. Mol Plant 6:1149–1162
Maltoni A, Mariotti B, Jacobs DF, Tani A (2012) Pruning methods to restore Catanea sativa stands attacked by Dryocosmus kuriphilus. New Forest 43(5–6):869–885
McKay JW (1942) Self-sterility in the Chinese Chestnut (Castanea mollissima). Proc Am Soc Hort Sci 41:156–161
Mert C, Soylu A (2006) Flower and stamen structures of male-fertile and male-sterile chestnut (Castanea sativa Mill.). J Am Soc Hort Sci 131(6):752–759
Mogensen HL (1973) Some histochemical, ultrastructural, and notritional aspects of the ovule of quercus gambelii. Am J Bot 60(1):48–54
Nakamura M (2001) Pollen tube growth and fertilization in Japanese chestnut (Castanea crenata Sieb. et Zucc.). J Jpn Soc Hort Sci 63:277–282 (In Japanese with English abstract)
Otles S, Selek I (2012) Phenolic compounds and antioxidant activities of chestnut (Castanea sativa Mill.) fruits. Qual Assur Saf Crop Foods 4(4):199–205
Raghavan V (2000) Pollen-pistil interactions and fertilization. Developmental biology of flowering plants. Springer Verlag, New York, pp 228–233
Reinhardt S, Ewald A, Hellwig F (2008) The correlation between ovule quality parameters and the seed yield at Cyclamen persicum Mill. J Appl Bot Food Qual 82:76–82
Ruane LG (2009) Post-pollination processes and non-random mating among compatible mates. Evolut Ecol Res 11:1031–1051
Shi Z, Stösser R (2005) Reproductive Biology of Chinese Chestnut (Castanea mollissima Blume). Eur J Hort Sci 70(2):96–103
Sogo A, Tobe H (2006a) Delayed fertilization and pollen-tube growth in pistils of Fagus japonica (Fagaceae). Am J Bot 93(12):1748–1756
Sogo A, Tobe H (2006b) The evolution of fertilization modes independent of the micropyle in Fagales and ‘pseudoporogamy’. Plant Syst Evol 259(1):73–80
Sogo A, Tobe H (2008) Mode of pollen tube growth in pistils of Ticodendron incognitum (Ticodendraceae, Fagales) and the evolution of chalazogamy. Bot J Linn Soc 157(4):621–631
Takaso T, Kimoto Y, Owens JN, Kono M, Mimura T (2013) Secretions from the female gametophyte and their role in spermatozoid induction in Cycas revoluta. Plant Reprod 26(1):17–23
Wang P, Changa CM, Watson ME, Dick WA, Chen Y, Hoitink HAJ (2004) Maturity indices for composed dairy and pig manures. Soil Biol Biochem 36(5):767–776
Wang R, Jia H, Wang JZ, Zhang ZX (2010) Flowering and pollination patterns of Magnolia denudata with emphasis on anatomical changes in ovule and seed development. Flora-Morphol Distrib Funct Ecol Plants 205(4):259–265
Wen J (1999) Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Ann Rev Ecol Syst, pp 421–455
Weterings K, Russell SD (2004) Experimental analysis of the fertilization process. Plant Cell Online 16(suppl 1):S107–S118
Yuan CQ, Sun YH, Li YF, Zhao KQ, Hu RY, Li Y (2014) Selection occurs within linear fruit and during the early stages of reproduction in Robinia pseudoacacia. BMC Evol Biol 14:53
Zou F, Guo SJ, Xie P, Xiong H, Lv WJ, Li GH (2014) Megasporogenesis and development of female gametophyte in Chinese Chestnut (Castanea mollissima) cultivar ‘Yanshanzaofeng’. Int J Agric Biol 16:1001–1005
Acknowledgments
The study was financed by the Chinese National Science and Technology Pillar Program (2013BAD14B04). The authors are highly grateful to those who help them to complete this work. We thank Dr. Chao Gao from Central South University of Forestry and Technology for assistance with the manuscript revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J. Carlson.
Rights and permissions
About this article
Cite this article
Fan, X., Yuan, D., Tang, J. et al. Sporogenesis and gametogenesis in Chinese chinquapin (Castanea henryi (Skam) Rehder & Wilson) and their systematic implications. Trees 29, 1713–1723 (2015). https://doi.org/10.1007/s00468-015-1251-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1251-y