Abstract
Key message
This study showed the negative effects of insect defoliation on the reproduction of canopy trees, where defoliation was not artificially manipulated but rather observed in a natural setting.
Abstract
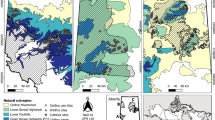
The gypsy moth (Lymantria diapar) is a serious defoliator with the ability to completely defoliate forest canopies. Although the negative effects of defoliation on tree reproduction have been revealed in studies with artificially manipulated defoliation, few studies have examined the effect of insect defoliation on the reproduction of canopy trees under natural condition. In this study, visual surveys were used to clarify the effect of gypsy moth defoliation on the production of acorns by oaks (Quercus spp.). Surveys were conducted in an outbreak year at 22 sites in central Japan (13 sites with Quercus crispula and nine sites with Q. serrata). Five of the Q. crispula sites were severely defoliated (mean defoliation ranged from 67 to 88 %), while the remainder of the Q. crispula sites and all of the Q. serrata sites were lightly defoliated (0–20 %). A negative effect of gypsy moth defoliation on acorn production was detected for Q. crispula. However, there was a synchronous decrease in acorn production from the previous year and crop levels were low at all sites regardless of the defoliation severity. The consistent low crop levels were likely the result of weather-related factors. Defoliation also negatively affected the production of acorns for Q. serrata; however, severe defoliation was not present at any Q. serrata sites. This study suggests that insect defoliation can affect forest ecosystem processes, such as the regeneration of host trees and the behavior of wildlife that depend on seed production, by reducing the reproductive potential of host trees.





Similar content being viewed by others
References
Akasofu Y (1973) Extraordinary outbreak of gypsy moth in Takaoka City. For Pests 22:214–217 (in Japanese)
Alalouni U, Schädler M, Brandl R (2013) Natural enemies and environmental factors affecting the population dynamics of the gypsy moth. J Appl Entomol 137:721–738. doi:10.1111/jen.12072
Aoki J (1974) Mixed infection of the gypsy moth, Lymantria dispar japonica MOTSCHULSKY (Lepidoptera: Lymantriidae), in a larch forest by Entomophthora aulicae (REICH.) SOROK. and Paecilomyces canadensis (VUILL.) BROWN et SMITH. Appl Entomol Zool 9:185–190. doi:10.1303/aez.9.185
Arimoto M, Iwaizumi R (2014) Identification of Japanese Lymantria species (Lepidoptera: Lymantriidae) based on PCR–RFLP analysis of mitochondrial DNA. Appl Entomol Zool 49:159–169. doi:10.1007/s13355-013-0235-x
Baker WL (1941) Effect of gypsy moth defoliation on certain forest trees. J Forestry 39:1017–1022
Bell JL, Whitmore RC (1997) Eastern towhee numbers increase following defoliation by gypsy moths. Auk 114:708–716. doi:10.2307/4089290
Campbell RW, Sloan RJ (1977) Forest stand responses to defoliation by the gypsy moth. For Sci Monogr 19:1–34
Collins S (1961) Benefits to understory from canopy defoliation by gypsy moth larvae. Ecology 42:836–838. doi:10.2307/1933521
Crawley MJ (1985) Reduction of oak fecundity by low-density herbivore populations. Nature 314:163–164. doi:10.1038/314163a0
de Beurs KM, Townsend PA (2008) Estimating the effect of gypsy moth defoliation using MODIS. Remote Sens Environ 112:3983–3990. doi:10.1016/j.rse.2008.07.008
Eisenbies MH, Davidson C, Johnson J, Amateis R, Gottschalk K (2007) Tree mortality in mixed pine–hardwood stands defoliated by the European gypsy moth (Lymantria dispar L.). For Sci 53:683–691
Eschtruth AK, Battles JJ (2014) Ephemeral disturbances have long-lasting impacts on forest invasion dynamics. Ecology 95:1770–1779. doi:10.1890/13-1980.1
Fajvan MA, Wood JM (1996) Stand structure and development after gypsy moth defoliation in the Appalachian Plateau. For Ecol Manage 89:79–88. doi:10.1016/S0378-1127(96)03865-0
Fajvan MA, Rentch J, Gottschalk K (2008) The effects of thinning and gypsy moth defoliation on wood volume growth in oaks. Trees 22:257–268. doi:10.1007/s00468-007-0183-6
Fukumoto H, Kajimura H (2011) Effects of asynchronous acorn production by co-occurring Quercus trees on resource utilization by acorn-feeding insects. J For Res 16:62–67. doi:10.1007/s10310-010-0208-7
Fukuyama K, Yamaguchi H, Koizumi C (1990) Comparative analysis of mortality factors of artificial larval populations of the gypsy moth, Lymantria dispar (Lepidotera: Lymantriidae) in Hokkaido: I. Mortality factors in five different types of forests. Appl Entomol Zool 25:205–213. doi:10.1303/aez.25.205
Furuno T (1964) On the feeding quantity of the gypsy moth (Lymantria dispar LINNE) and the camphor silk moth (Dictyoploca japonica BUTLER). J Jpn For Soc 46:14–19 (in Japanese with English summary)
Furuta K (1982) Natural control of Lymantria dispar L. (Lep., Lymantriidae) population at low density levels in Hokkaido (Japan). Zeitschrift für Angewandte Entomologie 93:513–522. doi:10.1111/j.1439-0418.1982.tb03629.x
Gandhi KJK, Herms DA (2010) Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions 12:389–405. doi:10.1007/s10530-009-9627-9
Gottschalk KW (1990) Gypsy moth effects on mast production. In: McGee CE (ed) Proceedings of the workshop: Southern Appalachian mast management. Knoxville, Tennessee, pp 42–50
Gottschalk KW, Colbert JJ, Feicht DL (1998) Tree mortality risk of oak due to gypsy moth. Eur J For Path 28:121–132. doi:10.1111/j.1439-0329.1998.tb01173.x
Grace JR (1986) The influence of gypsy moth on the composition and nutrient content of litter fall in a Pennsylvania oak forest. For Sci 32:855–870
Haynes KJ, Liebhold AM, Johnson DM (2012) Elevational gradient in the cyclicity of a forest-defoliating insect. Popul Ecol 54:239–250. doi:10.1007/s10144-012-0305-x
Higashiura Y (1980) Analysis of factors affecting bird predation on gypsy moth egg masses by using Holling’s disc-quation. Res Popul Ecol 22:147–162. doi:10.1007/BF02513542
Higashiura Y (1987) Larval densities and a life-table for the gypsy moth, Lymantria dispar, estimated using the head-capsule collection method. Ecol Entomol 12:25–30. doi:10.1111/j.1365-2311.1987.tb00981.x
Higashiura Y, Yamaguchi H, Ishihara M, Ono N, Tsukagoshi H, Yokobori S, Tokishita S, Yamagata H, Fukatsu T (2011) Male death resulting from hybridization between subspecies of the gypsy moth, Lymantria dispar. Heredity 106:603–613. doi:10.1038/hdy.2010.92
Hoch G (2005) Fruit-bearing branchlets are carbon autonomous in mature broad-leaved temperate forest trees. Plant Cell Environ 28:651–659. doi:10.1111/j.1365-3040.2004.01311.x
Hoch G, Siegwolf RW, Keel SG, Körner C, Han Q (2013) Fruit production in three masting tree species does not rely on stored carbon reserves. Oecologia 171:653–662. doi:10.1007/s00442-012-2579-2
Ichie T, Igarashi S, Yoshida S, Kenzo T, Masaki T, Tayasu I (2013) Are stored carbohydrates necessary for seed production in temperate deciduous trees? J Ecol 101:525–531. doi:10.1111/1365-2745.12038
Imada M, Nakai T, Nakamura T, Mabuchi T, Takahashi Y (1990) Acorn dispersal in natural stands of Mizunara (Quercus mongolica var. grosseserrata) for twenty years. J Jpn For Soc 72:426–430
Ishihama N, Yasaka M, Ohno Y, Hasui S, Nakagawa M, Takiya M (2011) Damage by outbreaks of gypsy moth in newly planted larch species plantations at Higashikagura, central Hokkaido. Trans Mtg Hokkaido Br Jpn For Soc 59:59–62 (in Japanese)
Janzen DH (1976) Effect of defoliation on fruit-bearing branches of the Kentucky coffee tree, Gymnoclaudus dioicus (Leguminosae). Am Midl Nat 95:474–478. doi:10.2307/2424414
Jedlicka J, Vandermeer J, Aviles-Vazquez K, Barros O, Perfecto I (2004) Gypsy moth defoliation of oak trees and a positive response of red maple and black cherry: An example of indirect interaction. Am Midl Nat 152:231–236. doi:10.1674/0003-0031(2004)152[0231:GMDOOT]2.0.CO;2
Jikumaru S, Sano T (2007) Distribution of late instar Lymantria dispar cadavers killed by Entomophaga maimaiga on trunks of several tree species in southwestern Japan. Can J Bot 85:25–30. doi:10.1139/b06-146
Kaitaniemi P, Neuvonen S, Nyyssönen T (1999) Effects of cumulative defoliations on growth, reproduction, and insect resistance in mountain birch. Ecology 80:524–532. doi:10.1890/0012-9658(1999)080[0524:EOCDOG]2.0.CO;2
Kamata N (2002) Outbreaks of forest defoliating insects in Japan, 1950–2000. Bull Entomol Res 92:109–117. doi:10.1079/BER2002159
Kasbohm JW (1994) Response of black bears to gypsy moth infestation in Shenandoah National Park, Virginia. PhD thesis, Virginia Polytechnic Institute and State University, Blacksburg, Virginia
Kasbohm JW, Kraus JG, Vaughan MR (1995) Food habits and nutrition of black bears during a gypsy moth infestation. Can J Zool 73:1771–1775. doi:10.1139/z95-208
Kasbohm JW, Vaughan MR, Kraus JG (1998) Black bear home range dynamics and movement patterns during a gypsy moth infestation. Ursus 10:259–267
Koenig WD, Knops JMH (2013) Large-scale spatial synchrony and cross-synchrony in acorn production by two California oaks. Ecology 94:83–93. doi:10.1890/12-0940.1
Koenig WD, Knops JMH (2014) Environmental correlates of acorn production by four species of Minnesota oaks. Popul Ecol 56:63–71. doi:10.1007/s10144-013-0408-z
Koenig WD, Knops JMH, Carmen WJ, Stanback MT, Mumme RL (1994) Estimating acorn crops using visual surveys. Can J For Res 24:2105–2112. doi:10.1139/x94-270
Kosola KR, Dickmann DI, Paul EA, Parry D (2001) Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 129:65–74. doi:10.1007/s004420100694
Koyama R (1954) Two epizootic diseases of gypsy moth. For Pests 3:296–298 (in Japanese)
Kozakai C, Yamazaki K, Nemoto Y, Nakajima A, Koike S, Abe S, Masaki T, Kaji K (2011) Effect of mast production on home range use of Japanese black bears. J Wildl Manage 75:867–875. doi:10.1002/jwmg.122
Kulman HM (1971) Effects of insect defoliation on growth and mortality of trees. Ann Rev Entomol 16:289–324. doi:10.1146/annurev.en.16.010171.001445
Lesnoff M, Lancelot R (2012) aod: Analysis of overdispersed data. R package version 1.3. http://cran.r-project.org/package=aod
Liebhold AM, Gottschalk KW, Muzika R, Montgomery ME, Young R, O’Day K, Kelley B (1995) Suitability of North American tree species to the gypsy moth: a summary of field and laboratory tests. General technical report NE-221. USDA Forest Service, Northeastern Forest Experiment Station, Radnor
Liebhold AM, Higashiura Y, Unno A (1998) Forest type affects predation on gypsy moth (Lepidoptera: Lymantriidae) pupae in Japan. Environ Entomol 27:858–862. doi:10.1093/ee/27.4.858
Liebhold A, Elkinton J, Williams D, Muzika RM (2000) What causes outbreaks of the gypsy moth in North America? Popul Ecol 42:257–266. doi:10.1007/PL00012004
Liebhold AM, Turcáni M, Kamata N (2008) Inference of adult female dispersal from the distribution of gypsy moth egg masses in a Japanese city. Agric For Entomol 10:69–73. doi:10.1111/j.1461-9563.2007.00359.x
Lovett GM, Canham CD, Arthur MA, Weathers KC, Fitzhugh RD (2006) Forest ecosystem responses to exotic pests and pathogens in eastern north America. Bioscience 56:395–405. doi:10.1641/0006-3568(2006)056[0395:FERTEP]2.0.CO;2
Maeto K, Ozaki K (2003) Prolonged diapause of specialist seed-feeders makes predator satiation unstable in masting of Quercus crispula. Oecologia 137:392–398. doi:10.1007/s00442-003-1381-6
Marquis RJ (1984) Leaf herbivores decrease fitness of a tropical plant. Science 226:537–539. doi:10.1126/science.226.4674.537
Massad TJ (2013) Ontogenetic differences of herbivory on woody and herbaceous plants: a meta-analysis demonstrating unique effects of herbivory on the young and the old, the slow and the fast. Oecologia 172:1–10. doi:10.1007/s00442-012-2470-1
May JD, Killingbeck KT (1995) Effects of herbivore-induced nutrient stress on correlates of fitness and on nutrient resorption in scrub oak (Quercus ilicifolia). Can J For Res 25:1858–1864. doi:10.1139/x95-200
Mizui N (1991) Classification of seed production based on the correlation between seed-weight and seed-number in deciduous broadleaved tree species. J Jpn For Soc 73:258–263 (in Japanese with English summary)
Mizutani M (2014) Gypsy moth outbreaks and their impacts on acorn production of Quercus crispula in Fukui Prefecture in 2013. Chubu For Res 62:63–66 (in Japanese)
Mizutani M, Nakajima H, Kodani J, Nogami T, Tada M (2013) Relationship between the acorn crops of Fagaceae trees and the mass intrusions of bears into residential areas in the Hokuriku region. J Jpn For Soc 95:76–82. doi:10.4005/jjfs.95.76 (in Japanese with English summary)
Morris WF, Hufbauer RA, Agrawal AA, Bever JD, Borowicz VA, Gilbert GS, Maron JL, Mitchell CE, Parker IM, Power AG, Torchin ME, Vázquez DP (2007) Direct and interactive effects of enemies and mutualists on plant performance: a meta-analysis. Ecology 88:1021–1029. doi:10.1890/06-0442
Muzika RM, Liebhold AM (1999) Changes in radial increment of host and nonhost tree species with gypsy moth defoliation. Can J For Res 29:1365–1373. doi:10.1139/x99-098
Naidoo R, Lechowicz MJ (2001) Effects of gypsy moth on radial growth of deciduous trees. For Sci 47:338–348
Nakajima H (2013) Estimation of beech (Fagus crenata) seed crops from female inflorescence scars: Relationship between seed crops and degree of intrusion into residential areas by Asiatic black bears in Toyama Prefecture, Japan. J Jpn For Soc 95:71–75. doi:10.4005/jjfs.95.71 (in Japanese with English summary)
Nakajima H (2015) Estimating sound seedfall density of Fagus crenata using a visual survey. J For Res 20:94–103. doi:10.1007/s10310-014-0440-7
Nakajima H, Ishida M (2014) Decline of Quercus crispula in abandoned coppice forests caused by secondary succession and Japanese oak wilt disease: Stand dynamics over twenty years. For Ecol Manage 334:18–27. doi:10.1016/j.foreco.2014.08.021
Nakajima H, Kume A, Ishida M, Ohmiya T, Mizoue N (2011) Evaluation of estimates of crown condition in forest monitoring: comparison between visual estimation and automated crown image analysis. Ann For Sci 68:1333–1340. doi:10.1007/s13595-011-0132-9
Obeso JR (1993) Does defoliation affect reproductive output in herbaceous perennials and woody plants in different ways? Func Ecol 7:150–155. doi:10.2307/2389881
Obeso JR (1998) Effects of defoliation and girdling on fruit production in Ilex aquifolium. Func Ecol 12:486–491. doi:10.1046/j.1365-2435.1998.00216.x
Oka T, Miura S, Masaki T, Suzuki W, Osumi K, Saitoh S (2004) Relationship between changes in beechnut production and Asiatic black bears in northern Japan. J Wildl Manage 68:979–986. doi:10.2193/0022-541X(2004)068[0979:RBCIBP]2.0.CO;2
Onodera K, Hara H (2011) Suitability of plant species as food for Asian gypsy moth larvae of the Hokkaido population. Bull Hokkaido For Res Inst 48:47–54 (in Japanese with English summary)
Palacio S, Hernández R, Maestro–Martínez M, Camarero JJ (2012) Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the re-growth ability of trees. Trees 26:1627–1640. doi:10.1007/s00468-012-0739-y
Piper FI, Fajardo A (2014) Foliar habit, tolerance to defoliation and their link to carbon and nitrogen storage. J Ecol 102:1101–1111. doi:10.1111/1365-2745.12284
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rieske LK, Dillaway DN (2008) Response of two oak species to extensive defoliation: Tree growth and vigor, phytochemistry, and herbivore suitability. For Ecol Manage 256:121–128. doi:10.1016/j.foreco.2008.04.015
Rockwood LL (1973) The effect of defoliation on seed production of six Costa Rican tree species. Ecology 54:1363–1369. doi:10.2307/1934200
Saitoh T, Vik JO, Stenseth NC, Takanishi T, Hayakashi S, Ishida N, Ohmori M, Morita T, Uemura S, Kadomatsu M, Osawa J, Maekawa K (2008) Effects of acorns abundance on density dependence in a Japanese wood mice (Apodemus speciosus) population. Popul Ecol 50:159–167. doi:10.1007/s10144-008-0076-6
Salleo S, Nardini A, Raimondo F, Gullo MAL, Pace F, Giacomich P (2003) Effects of defoliation caused by the leaf miner Cameraria ohridella on wood production and efficiency in Aesculus hippocastanum growing in north-eastern Italy. Trees 17:367–375. doi:10.1007/s00468-003-0247-1
Schowalter TD, Hargrove WW, Crossley DA (1986) Herbivory in forested ecosystems. Ann Rev Entomol 31:177–196. doi:10.1146/annurev.en.31.010186.001141
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149–151. doi:10.1126/science.217.4555.149
Shibata M, Tanaka H, Iida S, Abe S, Masaki T, Niiyama K, Nakashizuka T (2002) Synchronized annual seed production by 16 principal tree species in a temperate deciduous forest, Japan. Ecology 83:1727–1742. doi:10.1890/0012-9658(2002)083[1727:SASPBP]2.0.CO;2
Stephenson AG (1980) Fruit set, herbivory, fruit reduction, and the fruiting strategy of Catalpa speciosa (Bignoniaceae). Ecology 61:57–64. doi:10.2307/1937155
Wargo PM (1972) Defoliation-induced chemical changes in sugar maple roots stimulate growth of Armillaria mellea. Phytopathology 62:1278–1283. doi:10.1094/Phyto-62-1278
Wargo PM, Parker J, Houston DR (1972) Starch content in roots of defoliated sugar maple. For Sci 18:203–204
Wesołowski T, Rowiński P, Maziarz M (2015) Interannual variation in tree seed production in a primeval temperate forest: does masting prevail? Eur J For Res 134:99–112. doi:10.1007/s10342-014-0836-0
Williams DW, Fuester RW, Metterhouse WW, Balaam RJ, Bullock RH, Chianese RJ (1991) Oak defoliation and population density relationships for the gypsy moth (Lepidoptera: Lymantriidae). J Econ Entomol 84:1508–1514. doi:10.1093/jee/84.5.1508
Yasaka M, Takiya M, Watanabe I, Oono Y, Mizui N (2008) Variation in seed production among years and among individuals in 11 broadleaf tree species in northern Japan. J For Res 13:83–88. doi:10.1007/s10310-007-0052-6
Acknowledgments
I am grateful to my colleagues at Toyama Prefectural Government for their helpful support. This study was funded by Toyama Prefecture, Japan.
Conflict of interest
The author declares that he have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Koike.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakajima, H. Defoliation by gypsy moths negatively affects the production of acorns by two Japanese oak species. Trees 29, 1559–1566 (2015). https://doi.org/10.1007/s00468-015-1237-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-015-1237-9