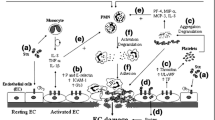
Abstract
The balance between the human body and surrounding microorganisms is crucial for homeostasis and health. A disturbance in host–pathogen interactions causes disease. Two important diseases of the kidney and urinary tract are directly caused by bacteria or bacterial toxins: urinary tract infection (UTI) and diarrhea-associated hemolytic uremic syndrome (HUS). In the majority of cases, UTIs are caused by bacteria ascending from the perineum through the urethra to the urinary tract. In contrast, HUS is caused by non-invasive bacteria, such as enterohemorrhagic Escherichia coli, which colonize the gut and do not enter the blood stream. In this latter case, the bacteria release Shiga toxin, which binds to blood cells and thus reaches the target organs, mainly kidneys. Interactions between Shiga toxin, blood cells and endothelial cells in the kidney lead to cell apoptosis and inflammation. Innate immunity and the antimicrobial peptide cathelicidin seem to play important roles in the pathogenesis of both UTI and HUS. Moreover, influencing cathelicidin production and release might offer new therapeutic and prophylactic strategies for both diseases.



Similar content being viewed by others
References
Savage DC (1977) Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31:107–133
Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science 307:1915–1920
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM (2005) Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol 6:57–64
Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R (1996) The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem 238:325–332
Kin NW, Chen Y, Stefanov EK, Gallo RL, Kearney JF (2011) Cathelin-related antimicrobial peptide differentially regulates T- and B-cell function. Eur J Immunol 41:3006–3016
Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454–457
Iimura M, Gallo RL, Hase K, Miyamoto Y, Eckmann L, Kagnoff MF (2005) Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. J Immunol 174:4901–4907
Snyder JA, Haugen BJ, Buckles EL, Lockatell CV, Johnson DE, Donnenberg MS, Welch RA, Mobley HL (2004) Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun 72:6373–6381
Tullus K, Jacobson SH, Katouli M, Brauner A (1991) Relative importance of eight virulence characteristics of pyelonephritogenic Escherichia coli strains assessed by multivariate statistical analysis. J Urol 146:1153–1155
Kaper JB, Nataro JP, Mobley HL (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2:123–140
Hooton TM, Scholes D, Stapleton AE, Roberts PL, Winter C, Gupta K, Samadpour M, Stamm WE (2000) A prospective study of asymptomatic sacteriuria in sexually active young women. N Engl J Med 343:992–997
Guyon F (1889) Note sur les conditions de réceptivité de l'appareil urinaire à l'invasion microbienne. Ann d mal d org génitourin 7:257–262
Rovsing T (1898) Ueber die Aetiologie, Pathogenese und Behandlung der septischen Infection en der Harnwege. Monatsberf Urol 3:503–535
Cox CE, Hinman F Jr (1961) Experiments with induced bacteriuria, vesical emptying and bacterial growth on the mechanism of bladder defense to infection. J Urol 86:739–748
Hess O (1913) Experimentelle Untersuchungen über die Bacterium coli-infektion der Harnorgane. Mitteil a d grenzeb d Med U Chir 26:135–175
Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Eden C (1983) Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun 40:273–283
Spencer JR, Schaeffer AJ (1986) Pediatric urinary tract infections. Urol Clin North Am 13:661–672
Jost SP, Gosling JA, Dixon JS (1989) The morphology of normal human bladder urothelium. J Anat 167:103–115
Chromek M, Brauner A (2008) Antimicrobial mechanisms of the urinary tract. J Mol Med 86:37–47
Apodaca G (2004) The uroepithelium: not just a passive barrier. Traffic 5:117–128
Parsons CL, Greenspan C, Moore SW, Mulholland SG (1977) Role of surface mucin in primary antibacterial defense of bladder. Urology 9:48–52
Grist M, Chakraborty J (1994) Identification of a mucin layer in the urinary bladder. Urology 44:26–33
N'Dow J, Jordan N, Robson CN, Neal DE, Pearson JP (2005) The bladder does not appear to have a dynamic secreted continuous mucous gel layer. J Urol 173:2025–2031
Gordon DM, Riley MA (1992) A theoretical and experimental analysis of bacterial growth in the bladder. Mol Microbiol 6:555–562
Norden CW, Green GM, Kass EH (1968) Antibacterial mechanisms of the urinary bladder. J Clin Invest 47:2689–2700
Spencer JD, Schwaderer AL, Wang H, Bartz J, Kline J, Eichler T, DeSouza KR, Sims-Lucas S, Baker P, Hains DS (2013) Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int 83:615–625
Spencer JD, Hains DS, Porter E, Bevins CL, DiRosario J, Becknell B, Wang H, Schwaderer AL (2012) Human alpha defensin 5 expression in the human kidney and urinary tract. PLoS One 7:e31712
Spencer JD, Schwaderer AL, Dirosario JD, McHugh KM, McGillivary G, Justice SS, Carpenter AR, Baker PB, Harder J, Hains DS (2011) Ribonuclease 7 is a potent antimicrobial peptide within the human urinary tract. Kidney Int 80:174–180
Valore EV, Park CH, Quayle AJ, Wiles KR, McCray PB Jr, Ganz T (1998) Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest 101:1633–1642
Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P (2003) Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14:2534–2543
Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276:7806–7810
Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI (1998) Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 42:2206–2214
Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A (2006) The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12:636–641
Nielsen KL, Dynesen P, Larsen P, Jakobsen L, Andersen PS, Frimodt-Moller N (2014) Role of urinary cathelicidin LL-37 and human beta-defensin 1 in uncomplicated Escherichia coli urinary tract infections. Infect Immun 82:1572–1578
Roberts RC, Mohr CD, Shapiro L (1996) Developmental programs in bacteria. Curr Top Dev Biol 34:207–257
Trautner BW, Darouiche RO (2004) Role of biofilm in catheter-associated urinary tract infection. Am J Infect Control 32:177–183
Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, Hultgren SJ (2004) Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci USA 101:1333–1338
Kai-Larsen Y, Luthje P, Chromek M, Peters V, Wang X, Holm A, Kadas L, Hedlund KO, Johansson J, Chapman MR, Jacobson SH, Romling U, Agerberth B, Brauner A (2010) Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog 6:e1001010
Shapiro JA (1998) Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol 52:81–104
Zogaj X, Nimtz M, Rohde M, Bokranz W, Romling U (2001) The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39:1452–1463
Barnhart MM, Chapman MR (2006) Curli biogenesis and function. Annu Rev Microbiol 60:131–147
Findlay B, Zhanel GG, Schweizer F (2010) Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob Agents Chemother 54:4049–4058
Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B (2006) Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci USA 103:9178–9183
Talukder P, Satho T, Irie K, Sharmin T, Hamady D, Nakashima Y, Kashige N, Miake F (2011) Trace metal zinc stimulates secretion of antimicrobial peptide LL-37 from Caco-2 cells through ERK and p38 MAP kinase. Int Immunopharmacol 11:141–144
Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH (2004) Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912
Sarker P, Ahmed S, Tiash S, Rekha RS, Stromberg R, Andersson J, Bergman P, Gudmundsson GH, Agerberth B, Raqib R (2011) Phenylbutyrate counteracts Shigella mediated downregulation of cathelicidin in rabbit lung and intestinal epithelia: a potential therapeutic strategy. PLoS One 6:e20637
Luthje P, Brauner H, Ramos NL, Ovregaard A, Glaser R, Hirschberg AL, Aspenstrom P, Brauner A (2013) Estrogen supports urothelial defense mechanisms. Sci Transl Med 5:190ra180
Hertting O, Holm A, Luthje P, Brauner H, Dyrdak R, Jonasson AF, Wiklund P, Chromek M, Brauner A (2010) Vitamin D induction of the human antimicrobial peptide cathelicidin in the urinary bladder. PLoS One 5:e15580
Nseir W, Taha M, Nemarny H, Mograbi J (2013) The association between serum levels of vitamin D and recurrent urinary tract infections in premenopausal women. Int J Infect Dis 17:e1121–e1124
Griffin PM, Ostroff SM, Tauxe RV, Greene KD, Wells JG, Lewis JH, Blake PA (1988) Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann Intern Med 109:705–712
Jerse AE, Yu J, Tall BD, Kaper JB (1990) A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA 87:7839–7843
Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201
Karpman D, Sartz L, Johnson S (2010) Pathophysiology of typical hemolytic uremic syndrome. Semin Thromb Hemost 36:575–585
Ståhl AL, Sartz L, Nelsson A, Bekassy ZD, Karpman D (2009) Shiga toxin and lipopolysaccharide induce platelet-leukocyte aggregates and tissue factor release, a thrombotic mechanism in hemolytic uremic syndrome. PLoS One 4:e6990
Obrig TG, Karpman D (2011) Shiga toxin pathogenesis: kidney complications and renal failure. Curr Top Microbiol Immunol 357:105–136
Pennington H (2010) Escherichia coli O157. Lancet 376:1428–1435
Louis P, Scott KP, Duncan SH, Flint HJ (2007) Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 102:1197–1208
Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Luhrs H, Gudmundsson GH (2003) Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut 52:735–741
Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx AP, Lebeer S, De Keersmaecker SC, Vanderleyden J, Hamalainen T, Laukkanen S, Salovuori N, Ritari J, Alatalo E, Korpela R, Mattila-Sandholm T, Lassig A, Hatakka K, Kinnunen KT, Karjalainen H, Saxelin M, Laakso K, Surakka A, Palva A, Salusjarvi T, Auvinen P, de Vos WM (2009) Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc Natl Acad Sci USA 106:17193–17198
Bishop JR, Gagneux P (2007) Evolution of carbohydrate antigens–microbial forces shaping host glycomes? Glycobiology 17:23R–34R
Johansson ME, Larsson JM, Hansson GC (2011) The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108[Suppl 1]:4659–4665
Jager S, Stange EF, Wehkamp J (2010) Antimicrobial peptides in gastrointestinal inflammation. Int J Inflamm 2010:910283
Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Putsep K, Andersson M (2008) Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57:764–771
Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF (2002) Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun 70:953–963
Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, Law I, Ho S, Ichikawa R, Zhao D, Xu H, Gallo R, Dempsey P, Cheng G, Targan SR, Pothoulakis C (2011) Cathelicidin signaling via the Toll-like receptor protects against colitis in mice. Gastroenterology 141:1852–1863
Calderon Toledo C, Rogers TJ, Svensson M, Tati R, Fischer H, Svanborg C, Karpman D (2008) Shiga toxin-mediated disease in MyD88-deficient mice infected with Escherichia coli O157:H7. Am J Pathol 173:1428–1439
Chromek M, Arvidsson I, Karpman D (2012) The antimicrobial peptide cathelicidin protects mice from Escherichia coli O157:H7-mediated disease. PLoS One 7:e46476
Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI (2000) The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med 342:1930–1936
Bitzan M (2009) Treatment options for HUS secondary to Escherichia coli O157:H7. Kidney Int 75[Suppl 112]:S62–66
Mukhopadhyay S, Linstedt AD (2012) Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science 335:332–335
Lapeyraque AL, Malina M, Fremeaux-Bacchi V, Boppel T, Kirschfink M, Oualha M, Proulx F, Clermont MJ, Le Deist F, Niaudet P, Schaefer F (2011) Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med 364:2561–2563
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chromek, M. The role of the antimicrobial peptide cathelicidin in renal diseases. Pediatr Nephrol 30, 1225–1232 (2015). https://doi.org/10.1007/s00467-014-2895-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-2895-3