Abstract
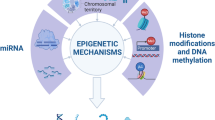
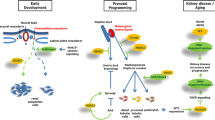
The notion that some adult diseases may have their origins in utero has recently captured scientists’ attention. Some of these effects persist across generations and may involve epigenetic mechanisms. Epigenetic modifications, DNA methylation together with covalent modifications of histones, alter chromatin density and accessibility of DNA to cellular machinery, modulating the transcriptional potential of the underlying DNA sequence. Here, we will discuss the different epigenetic modifications and their potential role in and contribution to renal disease development.



Similar content being viewed by others
References
Wood AJ, Oakey RJ (2006) Genomic imprinting in mammals: emerging themes and established theories. PLoS Genet 2:e147
Knoll JH, Nicholls RD, Magenis RE, Graham JM Jr, Lalande M, Latt SA (1989) Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet 32:285–290
Knoll JH, Nicholls RD, Magenis RE, Glatt K, Graham JM Jr, Kaplan L, Lalande M (1990) Angelman syndrome: three molecular classes identified with chromosome 15q11q13-specific DNA markers. Am J Hum Genet 47:149–154
Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S (2009) Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature 460:1132–1135
Yamanaka S (2009) Elite and stochastic models for induced pluripotent stem cell generation. Nature 460:49–52
Yamanaka S (2009) A fresh look at iPS cells. Cell 137:13–17
Feinberg AP, Tycko B (2004) The history of cancer epigenetics. Nat Rev Cancer 4:143–153
Dressler GR (2008) Epigenetics, development, and the kidney. J Am Soc Nephrol 19:2060–2067
Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J (2006) Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet 14:159–166
Kaati G, Bygren LO, Pembrey M, Sjostrom M (2007) Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet 15:784–790
Zandi-Nejad K, Luyckx VA, Brenner BM (2006) Adult hypertension and kidney disease: the role of fetal programming. Hypertension 47:502–508
Luyckx VA, Brenner BM (2005) Low birth weight, nephron number, and kidney disease. Kidney Int Suppl S68–S77
Alexander BT (2003) Intrauterine growth restriction and reduced glomerular number: role of apoptosis. Am J Physiol Regul Integr Comp Physiol 285:R933–R934
Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE (2006) Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69:671–678
McGraw M, Poucell S, Sweet J, Baumal R (1984) The significance of focal segmental glomerulosclerosis in oligomeganephronia. Int J Pediatr Nephrol 5:67–72
Hodgin JB, Rasoulpour M, Markowitz GS, D’Agati VD (2009) Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4:71–76
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group (2000) Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 342:381–389
Lopes-Virella MF, Carter RE, Gilbert GE, Klein RL, Jaffa M, Jenkins AJ, Lyons TJ, Garvey WT, Virella G (2008) Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diab Care 31:2006–2012
El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M (2008) Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205:2409–2417
Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A (2009) Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that co-exist on the lysine tail. Diabetes 58:1229–1236
White NH, Sun W, Cleary PA, Danis RP, Davis MD, Hainsworth DP, Hubbard LD, Lachin JM, Nathan DM (2008) Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthal 126:1707–1715
Dressler GR (2009) Advances in early kidney specification, development and patterning. Development 136:3863–3874
Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9:283–292
Patel SR, Kim D, Levitan I, Dressler GR (2007) The BRCT-domain containing protein PTIP links PAX2 to a histone H3, lysine 4 methyltransferase complex. Dev Cell 13:580–592
Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR (2009) Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462:315–322
Fazzari MJ, Greally JM (2004) Epigenomics: beyond CpG islands. Nat Rev 5:446–455
Chow J, Heard E (2009) X inactivation and the complexities of silencing a sex chromosome. Curr Opin Cell Biol 21:359–366
Agrelo R, Wutz A (2009) X inactivation and disease. Semin Cell Dev Biol 21:194–200
Reik W, Dean W, Walter J (2001) Epigenetic reprogramming in mammalian development. Science 293:1089–1093
Razin A (1998) CpG methylation, chromatin structure and gene silencing-a three-way connection. EMBO J 17:4905–4908
Ho KL, McNae IW, Schmiedeberg L, Klose RJ, Bird AP, Walkinshaw MD (2008) MeCP2 binding to DNA depends upon hydration at methyl-CpG. Mol Cell 29:525–531
Lasalle JM, Yasui DH (2009) Evolving role of MeCP2 in Rett syndrome and autism. Epigenomics 1:119–130
Bechtel W, McGoohan S, Zeisberg EM, Muller GA, Kalbacher H, Salant DJ, Muller CA, Kalluri R, Zeisberg M (2010) Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16:544–550
Einstein F, Thompson RF, Bhagat TD, Fazzari MJ, Verma A, Barzilai N, Greally JM (2010) Cytosine methylation dysregulation in neonates following intrauterine growth restriction. PLoS One 5:e8887
Marmorstein R, Trievel RC (2009) Histone modifying enzymes: structures, mechanisms, and specificities. Biochim Biophys Acta 1789:58–68
Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447:407–412
Guil S, Esteller M (2009) DNA methylomes, histone codes and miRNAs: tying it all together. Int J Biochem Cell Biol 41:87–95
Sayyed SG, Gaikwad AB, Lichtnekert J, Kulkarni O, Eulberg D, Klussmann S, Tikoo K, Anders HJ (2010) Progressive glomerulosclerosis in type 2 diabetes is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation and phosphorylation at serine 10. Nephrol Dial Transplant 25:1811–1817
Gaikwad AB, Sayyed SG, Lichtnekert J, Tikoo K, Anders HJ (2010) Renal failure increases cardiac histone h3 acetylation, dimethylation, and phosphorylation and the induction of cardiomyopathy-related genes in type 2 diabetes. Am J Pathol 176:1079–1083
Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R (2008) Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem 283:26771–26781
Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R (2008) Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA 105:9047–9052
Pentz ES, Lopez ML, Kim HS, Carretero O, Smithies O, Gomez RA (2001) Ren1d and Ren2 cooperate to preserve homeostasis: evidence from mice expressing GFP in place of Ren1d. Physiol Genomics 6:45–55
Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez ML (2009) CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol Heart Circ Physiol 296:H1255–H1262
Bjornsson HT, Fallin MD, Feinberg AP (2004) An integrated epigenetic and genetic approach to common human disease. Trends Genet 20:350–358
Wilson AG (2008) Epigenetic regulation of gene expression in the inflammatory response and relevance to common diseases. J Periodontol 79:1514–1519
Acknowledgements
We thank members of the Einstein Center for Epigenomics and the Susztak lab for the discussion. Our studies are supported by NIH 1R01DK087635 to KS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Answers:
1. c
2. e
3. d
4. e
5. d
Questions:
Questions:
Answers appear following the reference list.
-
1.
Epigenetics (nongenetic causes of a phenotype) may involve processes of:
-
a)
Genetic imprinting
-
b)
Histone methylation
-
c)
Cytosine methylation
-
d)
RNA interference
-
e)
All of the above
-
a)
-
2.
Euchromatin denotes the region in where DNA undergoes
-
a)
Transcriptional activation
-
b)
Demethylation
-
c)
Methylation
-
d)
Gene silencing
-
e)
a and b
-
a)
-
3.
Active histone marks (H3K4me3 and H3K36me3) refer to:
-
a)
Euchromatin
-
b)
Trimethylation
-
c)
Heterochromatin
-
d)
a and b
-
e)
All of the above
-
a)
-
4.
The epigenetic modifications play a role in following diseases and physiologic processes:
-
a)
Cancer
-
b)
Organ development
-
c)
Fetal programming
-
d)
Diabetes
-
e)
All of the above
-
a)
-
5.
Differences in clinical phenotype between Prader-Willi and Angelman syndromes result from:
-
a)
Histone acetylation
-
b)
DNA demethylation
-
c)
Expression of micro RNAs
-
d)
Genetic imprinting
-
e)
All of the above
-
a)
Rights and permissions
About this article
Cite this article
Woroniecki, R., Gaikwad, A.B. & Susztak, K. Fetal environment, epigenetics, and pediatric renal disease. Pediatr Nephrol 26, 705–711 (2011). https://doi.org/10.1007/s00467-010-1714-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-010-1714-8