Abstract
Background
Body core temperature is an important vital parameter during surgery and anaesthesia. It is influenced by several patient-related and surgery-related factors. Laparoscopy is considered beneficial in terms of a variety of parameters, for example, postoperative pain and length of hospital stay. Non-humidified, non-warmed insufflated CO2 applied during laparoscopy is standard of care. This prospective observational trial therefore evaluates the impact of non-humidified CO2 at room temperature on abdominal temperature and its correlation to body core temperature.
Methods
Seventy patients undergoing laparoscopic surgery were included in this prospective observational study. Temperature was measured oesophageal and abdominal before induction of anaesthesia (T1), right before skin incision (T2), 15 min, 30 min and 60 min after skin incision. All patients were treated according to actual guidelines for perioperative temperature measurement.
Results
Body core temperature and abdominal temperature correlated moderately (r = 0.6123; p < 0.0001). Bland–Altman plot for comparison of methods showed an average difference of 0.4 °C (bias − 0.3955; 95% agreement of bias from − 2.365 to 1.574). Abdominal temperature further decreased after establishing pneumoperitoneum (T2: 36.2 °C (35.9/36.4) to T5: 36.1 °C (35.6/36.4); p < 0.0001), whereas oesophageal temperature increased (T2: 36.2 °C (35.9/36.4) to 36.4 °C (36.0/36.7); p = 0.0296). Values of oesophageal and abdominal measurement points differed at T4 (36.3 °C (36.0/36.6) vs. 36.1 °C (35.4/36.6); p < 0.0001) and T5 (36.4 °C (36.0/36.7) vs. 36.1 °C (35.6/36.4) p = 0.0003).
Conclusion
This prospective observational trial shows the influence of insufflated, non-humidified carbon dioxide at room temperature on abdominal temperature during laparoscopic surgery. We show that carbon dioxide applied at these conditions decreases abdominal temperature and therefore might be a risk factor for perioperative hypothermia.
Similar content being viewed by others
Body core temperature is an important vital parameter during surgery and anaesthesia. Body core temperature influences several biochemical processes and physiological pathways. A Europe-wide survey in 2007 showed that in only 19% of surgeries under general anaesthesia, body temperature was documented [1]. However, perioperative temperature management is an important part of perioperative management.
A body core temperature below 36 °C is normally called “hypothermia”. Perioperative temperatures below this value reduce patients’ outcome due to negative effects on various physiological functions. Cardiovascular events like myocardial infarction, acute coronary syndrome (ACS) or arrhythmias increase significantly [2,3,4]. Likewise, blood coagulation is impaired, which leads to increased intraoperative blood losses and, as a consequence, increased transfusion requirements [4,5,6]. Perioperative hypothermia also impacts tissue oxygenation and leads to vasoconstriction resulting in an increased frequency of wound infections [7,8,9]. Last but not least, hypothermic patients are at discomfort in the recovery room. Hypothermia-related shivering increases oxygen consumption and as a consequence increases the risk of adverse cardiovascular events [10,11,12].
Body temperature during surgery is influenced by several factors which can be subdivided into different categories such as anaesthesia-related, surgery-related, environmental, or patient-related risk factors. Surgery-related risks include the surgical technique, the duration of surgery, the extent of the procedure, and the amount of irrigation fluid used [13]. Some studies showed that laparoscopic surgery can negatively influence body temperature by using carbon dioxide (CO2) at room temperature [14,15,16]. Nevertheless, laparoscopic surgery mostly is performed without pre-warming and humidifying CO2.
Therefore, the aim of this prospective, observational study was to investigate the influence of insufflated, non-humidified CO2 at room temperature on abdominal temperature compared to oesophageal temperature over time. Primary outcome was the correlation between both measurements.
Material and methods
The study was approved by the LMU Munich ethics committee (No. 17-143) and performed in accordance with the Declaration of Helsinki. This study also follows the CONSORT guidelines. Written informed consent was obtained from all patients participating in this prospective, observational study.
Inclusion criteria were planned laparoscopic surgery, age > 18 and patient’s written, informed consent. Exclusion criteria were age < 18, change to open surgery, pregnancy, combined epidural–general anaesthesia and patient’s denial.
Right before induction of anaesthesia, the first temperature measurement was made (T1). One min before surgical incision/establishing pneumoperitoneum the next temperature measurement was carried out (T2). Further temperature measurements took place at 15, 30 and 60 min after surgical incision (T3 and T5). Temperature was measured sublingually (Digitemp, servoprax GmbH: Wesel, Germany) before induction of anaesthesia (T1) and oesophageal from then on (T2-T5) according to recommended methods of temperature measurement [17]. Intra-abdominal measurement was done with a urinary catheter with integrated temperature measurement which was inserted through the camera trocar (T3-T5). It was placed right below the omentum and between the intestinal loops. Temperature was measured continuously except for the first measurement which was measured sublingually once.
Pre-warming of the patients was started immediately after arriving in the operating theatre using a whole-body blanket and warm air (Bair Hugger, 3 M, Maplewood, USA) (38 °C according to the manufacturer’s instructions). The patients were continuously and actively heated as required by the German guidelines during surgery [13, 17]. During surgery, patients received a preheated blanket over their legs and an actively warming blanket across thorax and upper extremities. Temperature in the operating theatre was set to 21 °C as recommended in the guideline and controlled by the anaesthetist.
Statistics
Sample size calculation was done with G*Power version 3.1 (HHU Düsseldorf, Germany) and based on an expected great effect of carbon dioxide (effect size dz = 0.3) at room temperature on abdominal temperature. A sample size of 70 patients was estimated to provide a power of 80% for detecting a statistically significant difference at a level of 0.05 (z-test; inequality of two dependent Pearson r’s).
Results
We included 70 patients undergoing laparoscopic surgery. Performed surgeries included appendectomy (n = 10), cholecystectomy (n = 20), adrenalectomy (n = 9), transabdominal preperitoneal hernia repair (TAPP; n = 8), sigmoid colectomy (n = 13), incisional hernia repair (n = 5), iliacal adenectomy (n = 1), adhesiolysis (n = 1), hepatic resection (liver cyst) (n = 1), implantation of abdominal dialysis catheter (n = 1) and hiatus hernia repair (n = 1). Detailed patient’s characteristics are displayed in Table 1.
Median blood loss and infused crystalloid were 20 ml (0/50) and 1000 ml (800/1500), respectively. None of the patients received colloids or packed red blood cells. All patients had general anaesthesia without epidural anaesthesia.
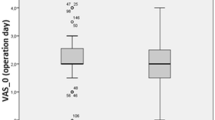
Before induction of anaesthesia, median temperature was 36.6 °C (36.2/36.9) and decreased despite active warming to 36.2 °C (35.9/36.4) right before incision (establishing pneumoperitoneum) (Fig. 1; p = 0.005). Temperature values remained below the initial temperature (T1) until 15 min after incision (36.2 °C (35.9/36.5); p = 0.006) for oesophageal temperature measurement (Fig. 1). After that, temperature did not differ compared to T1. Temperature measured abdominally remained below initial temperature values until 60 min after skin incision (pneumoperitoneum) (T3: 36.2 °C (35.5/36.6); T4: 36.1 °C (35.4/36.6); T5: 36.1 °C (35.6/36.4); all p < 0.0001). Compared to temperature right before pneumoperitoneum abdominal temperature further decreased (T2: 36.2 °C (35.9/36.4) to T5: 36.1 °C (35.6/36.4); p < 0.0001) whereas oesophageal temperature increased (T2: 36.2 °C (35.9/36.4) to 36.4 °C (36.0/36.7); p = 0.0296). Values of oesophageal and abdominal measurement points differed at T4 (36.3 °C (36.0/36.6) vs. 36.1 °C (35.4/36.6); p < 0.0001) and T5 (36.4 °C (36.0/36.7) vs. 36.1 °C (35.6/36.4) p = 0.0003).
Temperature over time course. T1: before anaesthesia induction (sublingual measurement); T2: 1 min before skin incision (establishing pneumoperitoneum; easophageal measurement); T3-5: 15, 30, 60 min after skin incision (oesophageal and abdominal measurements); $ vs. “anaesthesia induction” (oesophageal measurement). *vs. “anaesthesia induction” (abdominal measurement). #Abdominal vs. oesophageal measurement. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
We observed a moderate correlation (r = 0.6123, p < 0.0001) between intra-abdominal and oesophageal temperatures at all times of measurement. Additionally, we performed a Bland–Altman plot to compare both methods, which showed an average difference of 0.4 °C (bias − 0.3955; 95% agreement of bias from − 2.365 to 1.574).
Discussion
This prospective observational trial shows the influence of insufflated, non-humidified carbon dioxide at room temperature and on abdominal temperature during laparoscopic surgery. We were able to show that carbon dioxide applied under these conditions lessens abdominal temperature and therefore can be a risk factor for perioperative hypothermia. Correlation between oesophageal and abdominal temperature was moderate.
In the last decade, various risk factors for the development of perioperative hypothermia have been identified [13]. Both, patient-independent and patient-related factors influence the risk of hypothermia. Factors related to the patient are age, pre-existing low body temperature and patients with diabetic neuropathy [13]. Surgery-related factors include the duration and extent of surgery [13]. The influence of open versus laparoscopic surgery on body temperature is still not clear. Two randomized controlled studies investigated this aspect in the last years [18, 19]. Meta-analysis of these two studies favoured neither of both [13]. There was no significant difference intraoperatively concerning body temperature, but Nguyen et al. reported higher body temperatures at the post-anaesthesia care unit after laparoscopic and open gastric bypass (PACU) [19, 20]. However, considering the physiology of open surgery, it should have proven to be disadvantageous. The longer duration of surgery with laparoscopic technique was considered to be one reason for the equality results. Another reason which came into focus was CO2 used during laparoscopy. Normally it is used not humidified and at room temperature (20–22 °C). Some groups evaluated the effects of insufflated CO2 on intraoperative body temperature as well as on postoperative pain and length of hospital stay [15, 21]. The two meta-analyses of Dean et al. and Balayssac et al. were able to show positive effects of warmed, humidified CO2 compared to controls without warming [16, 21]. Jiang et al., however, demonstrated that warmed, humidified CO2 or combined forced air warming with CO2 at room temperature are equivalent [15].
In this context, abdominal temperature was measured in two studies and compared to the body core temperature, mostly measured in the oesophagus [19, 20]. In a small study (n = 20), Saad et al. evaluated intra-abdominal temperature and oesophageal temperature in groups with and without warmed CO2 but did not compare them. The study only compared groups concerning type of insufflation [20]. Nguyen et al. compared open and laparoscopic surgery in 101 patients, but only in 30 patients undergoing laparoscopic surgery intra-abdominal temperature was measured. Nevertheless, the group showed a significant difference between body core temperature (oesophagus) and abdominal temperature [19].
The data of our study support the results of Nguyen et al., but included a higher number of patients and external body warming was practiced according to the actual guidelines for perioperative temperature management [13, 17]. All patients were actively warmed by forced air warming pre-operatively and intraoperatively. Still, CO2 applied at room temperature significantly cooled down the abdomen. Although there already is evidence favouring warmed CO2 based on data measuring body core temperature, we wanted to demonstrate the negative effect of cool CO2 on abdominal temperature, which previously was shown only in studies with low numbers of patients. Hence, this study supports the actual results of Dean et al. and Balayssac et al., favouring warmed, humidified CO2 [16, 21].
Limitations
Our study has some limitations. First, different types of laparoscopic surgery were included in the study which may have had a (small) impact on the comparability the results. Second, the position of the oesophageal catheter was only verified by looking into the patients’ mouth and by groping. Thus, there might have been small differences concerning the exact intra-oesophageal position of the catheter between patients, which might have led to small deviations concerning temperature measurements. Fluid management (e.g. total volume and temperature of the volume) could have influenced the measurement. Nevertheless, all fluids were at room temperature and it was the same for all patients.
References
Torossian A (2007) Survey on intraoperative temperature management in Europe. Eur J Anaesthesiol 24:668–675
Elmore JR, Franklin DP, Youkey JR, Oren JW, Frey CM (1998) Normothermia is protective during infrarenal aortic surgery. J Vasc Surg 28:984–994
Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C (1997) Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA 277:1127–1134
Rajagopalan S, Mascha E, Na J, Sessler DI (2008) The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 108:71–77
Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A (1996) Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 347:289–292
Romlin B, Petruson K, Nilsson K (2007) Moderate superficial hypothermia prolongs bleeding time in humans. Acta Anaesthesiol Scand 51:198–201
Melling AC, Ali B, Scott EM, Leaper DJ (2001) Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet 358:876–880
Forbes SS, Eskicioglu C, Nathens AB, Fenech DS, Laflamme C, McLean RF, McLeod RS (2009) Evidence-based guidelines for prevention of perioperative hypothermia. J Am Coll Surg 209:492-503.e1
Kurz A, Sessler DI, Lenhardt R (1996) Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med 334:1209–1215
Macario A, Dexter F (2002) What are the most important risk factors for a patient’s developing intraoperative hypothermia? Anesth Analg 94:215–220
Alfonsi P, Nourredine KEA, Adam F, Chauvin M, Sessler DI (2003) Effect of postoperative skin-surface warming on oxygen consumption and the shivering threshold. Anaesthesia 58:1228–1234
Zwischenberger JB, Kirsh MM, Dechert RE, Arnold DK, Bartlett RH (1987) Suppression of shivering decreases oxygen consumption and improves hemodynamic stability during postoperative rewarming. Ann Thorac Surg 43:428–431
National Institute for Health and care Excellence (NICE) (2016) Hypothermia: prevention and management in adults having surgery. Available at https://www.nice.org.uk/guidance/cg65. Accessed 30 Jan 2020
Ryczek E, White J, Poole RL, Reeves NL, Torkington J, Carolan-Rees G (2019) Normothermic insufflation to prevent perioperative hypothermia and improve quality of recovery in elective colectomy patients: protocol for a randomized controlled trial. JMIR Res Protoc 8:e14533
Jiang R, Sun Y, Wang H, Liang M, Xie X (2019) Effect of different carbon dioxide (CO2) insufflation for laparoscopic colorectal surgery in elderly patients: a randomized controlled trial. Medicine 98:e17520
Dean M, Ramsay R, Heriot A, Mackay J, Hiscock R, Lynch AC (2017) Warmed, humidified CO2 insufflation benefits intraoperative core temperature during laparoscopic surgery: a meta-analysis. Asian J Endosc Surg 10:128–136
Torossian A, Becke K, Bein B, Bräuer A, Gantert D, Greif R, Höcker J, Horn E, Kimberger O, Klar E, Nuhn P, Ruchholtz S, Schwappach D, Welk I, Wulf H (2019) S3 Leitlinie “Vermeidung von perioperativer Hypothermie”. Available at https://www.awmf.org/uploads/tx_szleitlinien/001-018l_S3_Vermeidung_perioperativer_Hypothermie_2019-08.pdf. Accessed 30 Jan 2020
Danelli G, Berti M, Perotti V, Albertin A, Baccari P, Deni F, Fanelli G, Casati A (2002) Temperature control and recovery of bowel function after laparoscopic or laparotomic colorectal surgery in patients receiving combined epidural/general anesthesia and postoperative epidural analgesia. Anesth Analg 95:467–471
Nguyen NT, Fleming NW, Singh A, Lee SJ, Goldman CD, Wolfe BM (2001) Evaluation of core temperature during laparoscopic and open gastric bypass. Obes Surg 11:570–575
Saad S, Minor I, Mohri T, Nagelschmidt M (2000) The clinical impact of warmed insufflation carbon dioxide gas for laparoscopic cholecystectomy. Surg Endosc 14:787–790
Balayssac D, Pereira B, Bazin J-E, Le Roy B, Pezet D, Gagnière J (2017) Warmed and humidified carbon dioxide for abdominal laparoscopic surgery: meta-analysis of the current literature. Surg Endosc 31:1–12
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Philipp Groene, Ufuk Gündogar, Klaus Hofmann-Kiefer and Roland Ladurner have no conflicts of interest or financial ties to disclose. Philipp Groene received lecture fees by CSL Behring in the past.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Groene, P., Gündogar, U., Hofmann-Kiefer, K. et al. Influence of insufflated carbon dioxide on abdominal temperature compared to oesophageal temperature during laparoscopic surgery. Surg Endosc 35, 6892–6896 (2021). https://doi.org/10.1007/s00464-020-08196-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08196-x