Abstract
Background
Opportunities for surgical skills practice using high-fidelity simulation in the workplace are limited due to cost, time and geographical constraints, and accessibility to junior trainees. An alternative is needed to practise laparoscopic skills at home. Our objective was to undertake a systematic review of low-cost laparoscopic simulators.
Method
A systematic review was undertaken according to PRISMA guidelines. MEDLINE/EMBASE was searched for articles between 1990 and 2014. We included articles describing portable and low-cost laparoscopic simulators that were ready-made or suitable for assembly; articles not in English, with inadequate descriptions of the simulator, and costs >£1500 were excluded. Validation, equipment needed, cost, and ease of assembly were examined.
Results
Seventy-three unique simulators were identified (60 non-commercial, 13 commercial); 55 % (33) of non-commercial trainers were subject to at least one type of validation compared with 92 % (12) of commercial trainers. Commercial simulators had better face validation compared with non-commercial. The cost ranged from £3 to £216 for non-commercial and £60 to £1007 for commercial simulators. Key components of simulator construction were identified as abdominal cavity and wall, port site, light source, visualisation, and camera monitor. Laptop computers were prerequisite where direct vision was not used. Non-commercial models commonly utilised retail off-the-shelf components, which allowed reduction in costs and greater ease of construction.
Conclusion
The models described provide simple and affordable options for self-assembly, although a significant proportion have not been subject to any validation. Portable simulators may be the most equitable solution to allow regular basic skills practice (e.g. suturing, knot-tying) for junior surgical trainees.
Similar content being viewed by others
The use of laparoscopic surgery has become widely established in clinical practice, with the acquisition of laparoscopic skills now essential for surgical trainees. The technical skills required are, however, distinct from those needed for open surgery; depth perception is impaired due to visualisation on a two-dimensional screen, there is limited tactile feedback, and long laparoscopic instruments create a fulcrum effect and amplify tremor. There is a significant learning curve associated with laparoscopic surgery, and these skills cannot be easily learnt using the traditional apprentice model of surgical training [1].
Simulation is widely regarded as the way forward, and its use has been shown to improve laparoscopic surgical skills in trainees [2, 3]. Simulation offers the opportunity to improve technical skills in a structured, low-pressure environment outside of the operating theatre without risk to patient safety [4]. Different methods of simulation have been described, ranging from high-fidelity virtual reality systems and animal models to low-fidelity box trainers. Box trainers generally have a less realistic interface and are designed for the practice of generic skills required for laparoscopic surgery, such as instrument handling, cutting, and intracorporeal suturing. Virtual reality simulation uses computer-generated graphics and tactile feedback to recreate the operating environment, facilitating practice of procedural-specific skills as well as generic laparoscopic skills [5, 6]. Virtual reality systems are, however, very cost prohibitive and may be inaccessible to many trainees for regular personal use [7]. With the implementation of the European Working Time Directive, opportunities for surgical trainees to gain operative experience in the workplace have also become more limited [8]. A low-cost alternative is needed for trainees to be able to practise and develop their laparoscopic skills outside the workplace. Our objective was to undertake a systematic review of low-cost laparoscopic simulators suitable for home use.
Methods
A systematic review was undertaken according to PRISMA guidelines [9] to define the properties of low-cost laparoscopic simulators. MEDLINE and EMBASE databases were searched for articles on low-cost laparoscopic simulators published between January 1990 and August 2014. The search terms used were (laparoscopic or thoracoscopic or urological or gynaecological or gynaecological), (simulator or simulation or trainer or training), and (low-cost or home-made or inexpensive or DIY or cheap). Relevant articles from the search were identified by their titles and abstracts; the full paper was then assessed for inclusion. Reference lists for relevant articles were also examined to identify additional studies not identified by the original search.
Articles included were those describing low-cost laparoscopic simulators, which were ready-made or suitable for self-assembly. Articles not written in English, with inadequate descriptions of the simulator, and costs of >£1500 were excluded. The simulators described were categorised into commercial (commercially available or intended for commercial use) and non-commercial (intended for self-assembly). Validation, cost, equipment required, and ease of assembly were examined. For ease of comparison, simulator prices in other currencies were converted into British Pound Sterling using the exchange rate on 16 August 2014. We examined whether any form of validation had been described by the authors. The face validity of each simulator was also rated based on pre-defined criteria for the abdominal cavity and visualisation, giving a score between 0 and 6 (see Table 1).
Results
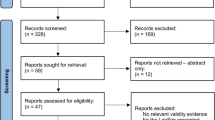
The results of the search are summarised in Fig. 1. 73 unique simulators were identified from 71 articles: 60 were non-commercial (Table 2) and 13 were commercial (Table 3); 55 % (33) of non-commercial trainers were subject to at least one type of validation compared with 92 % (12) of commercial trainers (Table 4). Commercial simulators were already constructed and ready to use, whereas non-commercial simulators required sourcing and self-assembly of materials. The key components required for non-commercial simulator construction were identified as abdominal cavity and wall, laparoscopic port site, light source, visualisation, and camera monitor.
Abdominal cavity and wall
Materials used to simulate the abdominal cavity aimed to prevent direct vision of the laparoscopic instruments; 68 % (41) of non-commercial simulators utilised off-the-shelf components for the abdomen, whilst 32 % (19) required a custom-made box. The commonest off-the-shelf component was a plastic storage box for the abdominal cavity, with the box lid serving as the abdominal wall [10–23]. Cardboard boxes were also commonly utilised [24–31].
Laparoscopic port site
The majority of non-commercial simulators (97 %, 58) required creating a hole in the abdominal wall material (by cutting, drilling or piercing) for the laparoscopic port site. Instruments could then be inserted directly into the cavity or through a trocar. Use of a flexible covering material, such as neoprene [13, 18], and ring reinforcement around the port site [13, 32–35] were also described as methods to increase simulator authenticity.
Primary light source
An adequate light source was required to visualise the interior of the abdominal cavity. External lighting was used for 38 % (23) of non-commercial simulators, particularly where boxes were made from a translucent material [11, 12, 17, 21] or had open sides [36–38]. This was useful in cost reduction, as no additional equipment was required to provide lighting in these cases. The built-in light source from the laparoscope itself provided lighting for 17 % (10) of simulators, desk lamps for 13 % (8), and light-emitting diodes (LED) for 8 % (5). Other lighting methods described included fluorescent lights [18, 34, 39], webcam in-built [40, 41], fibre optics [42], and torchlight [30].
Visualisation and camera monitor
Visualisation for non-commercial simulators was most commonly achieved using a webcam (37 %, 22) or laparoscope (22 %, 13). Other cameras types described included video cameras [29, 34, 43–45], digital cameras [24, 28, 46, 47], and tablet/smartphone cameras [30, 31, 37, 38]. Direct vision (full [10, 48] or unilaterally blinded [26]) and mirrors [23, 36] were non-electronic methods of visualisation described. Where electronic visualisation was used, a laptop computer, video monitor, tablet, or smartphone were prerequisite and not included in any cost estimates; this was true of both commercial and non-commercial simulators; 40 % (24) of models described use of a laptop/desktop computer screen and 38 % (23) described using a television or video monitor.
Cost
Forty-six percentage (26) of non-commercial and 54 % (6) of commercial simulators provided a figure for cost. For non-commercial, this was the cost of materials and assembly (e.g. custom-made parts); for commercial simulators, the cost represented the current or intended retail price. The cost ranged from £3 to £216 for non-commercial simulators and £60 to £1007 for commercial simulators. The cost of laparoscopic equipment (instruments and laparoscope) was not included in cost estimates for non-commercial simulators. However, a number of articles suggested that used or expired disposable instruments could be obtained from the operating department at no cost to the trainee [16, 23–26, 39, 40, 44]. Alternatively, they could also be obtained by donation from laparoscopic equipment manufacturers [15, 20, 26]. Electronic devices for visualisation (video monitor, laptop computer, tablet/smartphone) were not included in cost estimates for non-commercial simulators. Laparoscopic equipment and visualisation monitors were also not consistently included for commercial simulator model packages [49–52].
Face validity
Commercial simulators had better face validity than non-commercial simulators, with a median score of 5 compared to 3 (maximum 6). Commercial simulators tended to utilise higher-fidelity visualisation equipment, with a median visualisation score of B3 compared with B2 for non-commercial simulators. For the abdominal cavity, there was comparable face validity, with both groups having a median score of A2.
Discussion
Cost will undeniably be a key factor in the accessibility of a simulator model. Many articles omitted cost estimates, so there is difficulty in making a true cost comparison between commercial and non-commercial simulators available. Although there is an overlap in the price range, non-commercial models appear to be able to achieve a lower cost than commercial ones, with the lowest reported figure being $5 (£3) compared to $100 (£60) for a commercial model [37, 53]. This difference could be due to commercial models factoring in a profit margin and assembly fee in addition to the value of the raw materials. Moreover, commercial models will usually include expensive laparoscopic instruments in the cost, which could potentially be obtained cost-free when self-assembling [16, 23–26, 44].
Non-commercial models commonly utilised off-the-shelf components—a potentially a cost-reductive strategy, as custom-made parts could incur a greater expense. In particular, the use of a translucent plastic box provided a sturdy frame and utilised external lighting, negating the need for an additional light source inside the box [11, 12, 17, 21]. Visualisation using a webcam and computer offered an inexpensive solution, as they can be obtained cheaply. With computer ownership being widespread [54], it can be assumed that most trainees have access to a computer at home. Many trainees may also own a tablet computer. Tablet-based simulation could provide a video feed more comparable in quality to a laparoscope than a budget webcam [31]. Using a tablet or smartphone, where the screen and camera are on the same device, may also be easier to assemble. However, adjustment of camera position would be more difficult.
Commercial simulators, although seemingly costlier in comparison, do have the advantage that they come assembled and ready to use, with more models having undergone some form of validation. However, the appropriateness of the validation methods undertaken are not easily assessed, and only models from established industry suppliers appear to have undergone more extensive validation [50, 55]. In terms of face validity, commercial simulators largely seem to have better face validity, particularly as laparoscopes are more frequently used for visualisation, allowing realistic image quality and camera motion. A laparoscope may be difficult to obtain at a reasonable cost; an alternative may be to use a small camera mounted on a plastic pipe, which also allows adjustment of the operative field view [11, 16, 17]. The ideal simulator would have a highly realistic user interface and allow development of both the technical and non-technical skills required for laparoscopic surgery. The simulators examined in this review chiefly aim to develop basic laparoscopic skills such as instrument handling and cutting; therefore, a highly realistic user interface, as in virtual reality simulators, may be superfluous to requirements. However, use of lower-fidelity simulators does not preclude the development of non-technical skills. For example, the simulator could be incorporated into an operating theatre environment with other team members present, where trainees could be observed and assessed on emergency or elective scenarios.
Of course, simply having access to a simulator does not equate to improvement in surgical skill. Regular use of the trainer with feedback from a supervisor would be ideal. Simulator training could take place during the normal working day with allocated practice time, or this could be done at leisure at home.
Conclusion
The models described provide simple and affordable options for self-assembly, although a significant proportion has not been subject to any validation. Whilst simulation cannot replace operating theatre experience, portable simulators may be the most equitable solution to allow regular basic skills practice (e.g. intra-corporeal suturing, knot-tying) for junior surgical trainees.
References
Aggarwal R, Moorthy K, Darzi A (2004) Laparoscopic skills training and assessment. Br J Surg 91:1549–1558
Nagendran M, Gurusamy KS, Aggarwal R, Loizidou M, Davidson BR (2013) Virtual reality training for surgical trainees in laparoscopic surgery. Cochrane Database Syst Rev (8):CD006575. doi:10.1002/14651858.CD006575.pub3
Zendejas B, Brydges R, Hamstra SJ, Cook DA (2013) State of the evidence on simulation-based training for laparoscopic surgery: a systematic review. Ann Surg 257:586–593. doi:10.1097/SLA.0b013e318288c40b
Gaba DM (2004) The future vision of simulation in health care. Qual Saf Health Care 13(Suppl 1):i2–i10
Undre S, Darzi A (2007) Laparoscopy simulators. J Endourol 21:274–279
Dunkin B, Adrales G, Apelgren K, Mellinger J (2007) Surgical simulation: a current review. Surg Endosc 21:357–366
Schijven M, Jakimowicz J (2003) Virtual reality surgical laparoscopic simulators. Surg Endosc Other Interv Tech 17:1943–1950
Fitzgerald J, Caesar B (2012) The European working time directive: a practical review for surgical trainees. Int J Surg 10:399–403
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. doi:10.1136/bmj.b2535
Mughal M (1992) A cheap laparoscopic surgery trainer. Ann R Coll Surg Engl 74:256–257
Pokorny MR, McLaren SL (2004) Inexpensive home-made laparoscopic trainer and camera. ANZ J Surg 74:691–693
Beatty JD (2005) How to build an inexpensive laparoscopic webcam-based trainer. BJU Int 96:679–682
Ricchiuti D, Ralat DA, Evancho-Chapman M, Wyneski H, Cerone J, Wegryn JD (2005) A simple cost-effective design for construction of a laparoscopic trainer. J Endourol 19:1000–1005
Bell R, Maseelall P, Fanning J, Fenton B, Flora R (2007) A laparoscopic simulator tool for objective measurement of residents’ laparoscopic ability. JSLS 11:470–473
Raptis D, Mouzaki K, Gore D (2008) Technical notes and tips: DIY laparoscopic kit. Ann R Coll Surg Engl 90:167
Al-Abed Y, Cooper DG (2009) A novel home laparoscopic simulator. J Surg Educ 66:1–2
Rivas AM, Vilanova AC, Pereferrer FS, González MH, del Castillo Déjardin D (2010) Low cost simulator for acquiring basic laparoscopic skills. Cirugía Española (English Edition) 87:26–32
Khine M, Leung E, Morran C, Muthukumarasamy G (2011) Homemade laparoscopic simulators for surgical trainees. Clin Teach 8:118–121
Kiely DJ, Stephanson K, Ross S (2011) Assessing image quality of low-cost laparoscopic box trainers: options for residents training at home. Simul Healthc 6:292–298. doi:10.1097/SIH.0b013e31821cdb68
Kobayashi SA, Jamshidi R, O’Sullivan P, Palmer B, Hirose S, Stewart L, Kim EH (2011) Bringing the skills laboratory home: an affordable webcam-based personal trainer for developing laparoscopic skills. J Surg Educ 68:105–109
Moreira-Pinto J, Silva JG, Ribeiro de Castro JL, Correia-Pinto J (2013) Five really easy steps to build a homemade low-cost simulator. Surg Innov 20:95–99. doi:10.1177/1553350612440508
Beard JH, Akoko L, Mwanga A, Mkony C, O’Sullivan P (2014) Manual laparoscopic skills development using a low-cost trainer box in Tanzania. J Surg Educ 71:85–90
Walczak DA, Piotrowski P, Jędrzejczyk A, Pawełczak D, Pasieka Z (2014) A laparoscopic simulator—maybe it is worth making it yourself. Wideochir Inne Tech Maloinwazyjne 9(3):380–386. doi:10.5114/wiitm.2014.44139
Blacker AJ (2005) How to build your own laparoscopic trainer. J Endourol 19:748–752
Chung SY, Landsittel D, Chon CH, Ng CS, Fuchs GJ (2005) Laparoscopic skills training using a webcam trainer. J Urol 173:180–183
Chandrasekera SK, Donohue JF, Orley D, Barber NJ, Shah N, Bishai PM, Muir GH (2006) Basic laparoscopic surgical training: examination of a low-cost alternative. Eur Urol 50:1285–1291
Mir IS, Mohsin M, Malik A, Shah AQ, Majid T (2008) A structured training module using an inexpensive endotrainer for improving the performance of trainee surgeons. Trop Doct 38:217–218. doi:10.1258/td.2008.070359
Singh I, Panesar N, Haq A (2009) Blue Peter: on a shoe string budget for laparoscopic training. J Postgrad Med 55:233–234. doi:10.4103/0022-3859.57396
Rabie M (2010) Acquiring laparoscopic suturing skills using a homemade trainer. Eur Surg 42:149–151
Alfa-Wali M, Antoniou A (2011) Eco-friendly laparoscopic home trainer. Simul Healthc 6:176–179. doi:10.1097/SIH.0b013e318208549b
Bahsoun AN, Malik MM, Ahmed K, El-Hage O, Jaye P, Dasgupta P (2013) Tablet based simulation provides a new solution to accessing laparoscopic skills training. J Surg Educ 70:161–163
Sackier JM, Berci G, Paz-Partlow M (1991) A new training device for laparoscopic cholecystectomy. Surg Endosc 5:158–159
Majeed AW, Reed MW, Johnson AG (1992) Simulated laparoscopic cholecystectomy. Ann R Coll Surg Engl 74:70–71
Martinez AM, Espinoza DL (2007) Novel laparoscopic home trainer. Surg Laparosc Endosc Percutan Tech 17:300–302. doi:10.1097/SLE.0b013e31805d091d
Dennis R (2008) A simple and cheap home built laparoscopic trainer. J Minim Access Surg 4:88
Robinson JK, Kushner DM (2006) Development and validation of a home-based, mirrored, gynecologic laparoscopy trainer. J Minim Invasive Gynecol 13:102–107
Ruparel RK, Brahmbhatt RD, Dove JC, Hutchinson RC, Stauffer JA, Bowers SP, Richie E, Lannen AM, Thiel DD (2014) “iTrainers”–novel and inexpensive alternatives to traditional laparoscopic box trainers. Urology 83:116–120
Escamirosa Fernando P, Flores Ricardo O, Martínez Arturo M (2014) How to build a portable laparoscopic trainer for smartphones and tablets. J Laparoendosc Adv Surg Tech B. doi:10.1089/vor.2014.0200
Sparks D, Chase D, Lee W (2008) An inexpensive solution for laparoscopic simulation. OPUS 12:1–3
Helmy S, El-Shenoufy A (2009) Development of laparoscopic skills using a new inexpensive webcam trainer. J Biol Sci 9:766–771
Omokanye L, Olatinwo A, Salaudeen A, Balogun O, Saidu R (2013) An improvised endotrainer for low resource settings. Res J Health Sci 1:2360–7793
Dhariwal AK, Prabhu RY, Dalvi AN, Supe AN (2007) Effectiveness of box trainers in laparoscopic training. J Minim Access Surg 3:57–63. doi:10.4103/0972-9941.33274
Gue S (1995) Home-made videoscopic trainer for operative laparoscopic surgery. Aust N Z J Surg 65:820–821
Lee AC (2003) A homemade minimal access surgical skills station. Pediatric Endosurg Innov Tech 7:273–277
Griffin S, Kumar A, Burgess N, Donaldson P (2006) Development of laparoscopic suturing skills: a prospective trial. J Endourol 20:144–148
Haveran LA, Novitsky YW, Czerniach DR, Kaban GK, Taylor M, Gallagher-Dorval K, Schmidt R, Kelly JJ, Litwin DE (2007) Optimizing laparoscopic task efficiency: the role of camera and monitor positions. Surg Endosc 21:980–984
Pawar DS, Singh SK, Benjwal S, Kumari I (2010) A novel idea of using digital camera for laparoscopy training in urology. Urol J 7:56–58
Sharpe BA, MacHaidze Z, Ogan K (2005) Randomized comparison of standard laparoscopic trainer to novel, at-home, low-cost, camera-less laparoscopic trainer. Urology 66:50–54
Hruby GW, Sprenkle PC, Abdelshehid C, Clayman RV, McDougall EM, Landman J (2008) The EZ Trainer: validation of a portable and inexpensive simulator for training basic laparoscopic skills. J Urol 179:662–666
Hennessey IA, Hewett P (2013) Construct, concurrent, and content validity of the eoSim laparoscopic simulator. J Laparoendosc Adv Surg Tech 23:855–860
Xiao DJ, Albayrak A, Buzink SN, Jakimowicz J, Goossens RHM (2013) A newly designed portable laparoscopic trainer based on ergonomic guidelines. Surg Endosc Other Interv Tech 27:S5
Xiao D, Jakimowicz JJ, Albayrak A, Buzink SN, Botden SM, Goossens RH (2014) Face, content, and construct validity of a novel portable ergonomic simulator for basic laparoscopic skills. J Surg Educ 71:65–72
Yoon R, del Junco M, Kaplan A, Okhunov Z, Bucur P, Hofmann M, Alipanah R, McDougall EM, Landman J (2015) Development of a novel iPad-based laparoscopic trainer and comparison with a standard laparoscopic trainer for basic laparoscopic skills testing. J Surg Educ 72:41–46
Office for National Statistics (2014) Internet access—households and individuals 2014. http://www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/bulletins/internetaccesshouseholdsandindividuals/2015-08-06
Nakamura LY, Martin GL, Fox JC, Andrews PE, Humphreys M, Castle EP (2012) Comparing the portable laparoscopic trainer with a standardized trainer in surgically naive subjects. J Endourol 26:67–72
Chung J, Sackier J (1998) A method of objectively evaluating improvements in laparoscopic skills. Surg Endosc 12:1111–1116
Shapiro S, Paz-Partlow M, Daykhovsky L, Gordon L (1996) The use of a modular skills center for the maintenance of laparoscopic skills. Surg Endosc 10:816–819
Hasson HM, Aruna Kumari NV, Eekhout J (2001) Training simulator for developing laparoscopic skills. JSLS 5:255–265
Do AT, Cabbad MF, Kerr A, Serur E, Robertazzi RR, Stankovic MR (2006) A warm-up laparoscopic exercise improves the subsequent laparoscopic performance of Ob-Gyn residents: a low-cost laparoscopic trainer. JSLS 10:297–301
Nataraja R, Ade-Ajayi N, Holak K, Arbell D, Curry J (2006) Pilot study of new training model for laparoscopic surgery. Pediatr Surg Int 22:546–550
Nataraja R, Ade-Ajayi N, Curry J (2006) Surgical skills training in the laparoscopic era: the use of a helping hand. Pediatr Surg Int 22:1015–1020
Clevin L, Grantcharov TP (2008) Does box model training improve surgical dexterity and economy of movement during virtual reality laparoscopy? A randomised trial. Acta Obstet Gynecol Scand 87:99–103
Jain M, Tantia O, Khanna S, Sen B, Kumar Sasmal P (2009) Hernia endotrainer: results of training on self-designed hernia trainer box. J Laparoendosc Adv Surg Tech 19:535–540
Jaber N (2010) The basket trainer: a homemade laparoscopic trainer attainable to every resident. J Minim Access Surg 6:3–5. doi:10.4103/0972-9941.62525
Oliver J, Carty N, Wakefield C (2010) Low-cost model for laparoscopic appendicectomy in a webcam simulator. Bull R Coll Surg Engl 92:122–125
Ramalingam M, Senthil K, Murugesan A, Pai MG (2010) Cost reductive laparoendoscopic single site surgery endotrainer and animal lab training-our methodology. Diagn Ther Endosc 2010:598165. doi:10.1155/2010/598165
Afuwape O (2012) An affordable laparoscopic surgery trainer for trainees in poor resource settings. West Afr J Med 31(1):63–65
Akdemir A, Şendağ F, Öztekin MK (2014) Laparoscopic virtual reality simulator and box trainer in gynecology. Int J Gynecol Obstet 125:181–185
Hennessey IA (2012) How to make a portable laparoscopic simulator. J Laparoendosc Adv Surg Tech 22
Smith MD, Norris JM, Kishikova L, Smith DP (2013) Laparoscopic simulation for all: two affordable, upgradable, and easy-to-build laparoscopic trainers. J Surg Educ 70:217–223
Wong J, Bhattacharya G, Vance SJ, Bistolarides P, Merchant AM (2013) Construction and validation of a low-cost laparoscopic simulator for surgical education. J Surg Educ 70:443–450
Derossis AM, Fried GM, Abrahamowicz M, Sigman HH, Barkun JS, Meakins JL (1998) Development of a model for training and evaluation of laparoscopic skills. Am J Surg 175:482–487
Keyser EJ, Derossis AM, Antoniuk M, Sigman HH, Fried GM (2000) A simplified simulator for the training and evaluation of laparoscopic skills. Surg Endosc 14:149–153
Scott DJ, Bergen PC, Rege RV, Laycock R, Tesfay ST, Valentine RJ, Euhus DM, Jeyarajah DR, Thompson WM, Jones DB (2000) Laparoscopic training on bench models: better and more cost effective than operating room experience? J Am Coll Surg 191:272–283
Adrales G, Chu U, Witzke D, Donnelly M, Hoskins D, Mastrangelo M, Gandsas A, Park A (2003) Evaluating minimally invasive surgery training using low-cost mechanical simulations. Surg Endosc Other Interv Tech 17:580–585
Adrales G, Chu U, Hoskins J, Witzke D, Park A (2004) Development of a valid, cost-effective laparoscopic training program. Am J Surg 187:157–163
Waseda M, Inaki N, Mailaender L, Buess G (2005) An innovative trainer for surgical procedures using animal organs. Minim Invasive Ther Allied Technol 14:262–266
Dayan AB, Ziv A, Berkenstadt H, Munz Y (2008) A simple, low-cost platform for basic laparoscopic skills training. Surg Innov 15:136–142
Boon JR, Salas N, Avila D, Boone TB, Lipshultz LI, Link RE (2008) Construct validity of the pig intestine model in the simulation of laparoscopic urethrovesical anastomosis: tools for objective evaluation. J Endourol 22:2713–2716
Singh PB, Saw NK, Mokete M, Martin FL, Matanhelia SS (2008) An integrated laparoscopic simulator (i-Sim™) to develop surgical skills outside the operating theatre: a novel means to improve training facilities in the UK. Int J Surg 6:64–70
Hull L, Kassab E, Arora S, Kneebone R (2010) Increasing the realism of a laparoscopic box trainer: a simple, inexpensive method. J Laparoendosc Adv Surg Tech Part A 20:559–562
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Miss. Mimi M Li and Mr. Joseph George have no conflicts of interest or financial ties to disclose.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, M.M., George, J. A systematic review of low-cost laparoscopic simulators. Surg Endosc 31, 38–48 (2017). https://doi.org/10.1007/s00464-016-4953-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-4953-3