Abstract
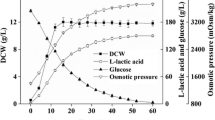
In Saccharomyces cerevisiae, proline is a stress protectant interacting with other substrate uptake systems against oxidative stress under low pH conditions. In this study, we performed metabolomics analysis to investigate the response associated with an increase in cell growth rates and maximum densities when cells were treated with proline under normal and acid stress conditions. Metabolome data show that concentrations of components of central metabolism are increased in proline-treated S. cerevisiae. No consumption of proline was observed, suggesting that proline does not act as a nutrient but regulates metabolic state and growth of cells. Treatment of lactic acid-producing yeast with proline during lactic acid bio-production improved growth rate and increased the final concentration of lactic acid.



Similar content being viewed by others
References
Mager WH, Moradas Ferreira P (1993) Stress response in yeast. Biochem J 290:1–13
Abbott DA, Suir E, Duong GH, de Hulster E, Pronk JT, van Maris AJ (2009) Catalase overexpression reduces lactic acid-induced oxidative stress in Saccharomyces cerevisiae. Appl Environ Microbiol 75:2320–2325
Valli M, Sauer M, Branduardi P, Borth N, Porro D, Mattanovich D (2006) Improvement of lactic acid production in Saccharomyces cerevisiae by cell sorting for high intracellular pH. Appl Environ Microbiol 72:5492–5499
Lohmeier-Vogel EM, Sopher CR, Lee H (1998) Intracellular acidification as a mechanism for the inhibition by acid hydrolysis-derived inhibitors of xylose fermentation by yeasts. J Ind Microbiol Biotechnol 20:75–81
Matsushika A, Sawayama S (2012) Characterization of a recombinant flocculent Saccharomyces cerevisiae strain that co-ferments glucose and xylose II. Influence of pH and acetic acid on ethanol production. Appl Biochem Biotechnol 168:2094–2104
Piper PW (1999) Yeast superoxide dismutase mutants reveal a pro-oxidant action of weak organic acid food preservatives. Free Radic Biol Med 27:1219–1227
Ali MA, Konishi T (1998) Enhancement of hydroxyl radical generation in the Fenton reaction by alpha-hydroxy acid. Biochem Mol Biol Int 46:137–145
Ali MA, Yasui F, Matsugo S, Konishi T (2000) The lactate-dependent enhancement of hydroxyl radical generation by the Fenton reaction. Free Radic Res 32:429–438
Grant RL, Acosta D (1997) Ratiometric measurement of intracellular pH of cultured cells with BCECF in a fluorescence multi-well plate reader. In Vitro Cell Dev Biol Anim 33:256–260
Stephen DWS, Jamieson DJ (1997) Amino acid-dependent regulation of the Saccharomyces cerevisiae GSH1 gene by hydrogen peroxide. Mol Microbiol 23:203–210
Hiltunen JK, Mursula AM, Rottensteiner H, Wierenga RK, Kastaniotis AJ, Gurvitz A (2003) The biochemistry of peroxisomal β-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 27:35–64
Grant CM, Perrone G, Dawes IW (1998) Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun 253:893–898
Reinders J, Wagner K, Zahedi RP, Stojanovski D, Eyrich B, van der Laan M, Rehling P, Sickmann A, Pfanner N, Meisinger C (2007) Profiling phosphoproteins of yeast mitochondria reveals a role of phosphorylation in assembly of the ATP synthase. Mol Cell Proteomics 6:1896–1906
Schmidt M, Akasaka K, Messerly JT, Boyer MP (2012) Role of Hog1, Tps1 and Sod1 in boric acid tolerance of Saccharomyces cerevisiae. Microbiology 158:2667–2678
Tsang CK, Lui Y, Thomas J, Zhang Y, Zheng XFS (2014) Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat Commun 5:3446
Garrido EO, Grant CM (2002) Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol Microbiol 43:993–1003
Nugroho RH, Yoshikawa K, Shimizu H (2015) Metabolomic analysis of acid stress response in Saccharomyces cerevisiae. J Biosci Bioeng 120:396–404
Terao Y, Nakamori S, Takagi H (2003) Gene dosage effect of l-proline biosynthetic enzymes on l-proline accumulation and freeze tolerance in Saccharomyces cerevisiae. Appl Environ Microbiol 69:6527–6532
Nomura M, Takagi H (2004) Role of the yeast acetyltransferase Mpr1 in oxidative stress regulation of oxygen reactive species caused by a toxic proline catabolism intermediate. Proc Natl Acad Sci USA 101:12616–12621
Nasuno R, Hirano Y, Itoh T, Hakoshima T, Hibi T, Takagi H (2013) Structural and functional analysis of the yeast N-acetyltransferase Mpr1 involved in oxidative stress tolerance via proline metabolism. Proc Natl Acad Sci USA 110:11821–11826
Signorelli S, Coitiño EL, Borsani O, Monza J (2014) Molecular mechanisms for the reaction between ·OH radicals and proline insights on the role as reactive oxygen species scavenger in plant stress. J Phys Chem B 118:37–47
Ida Y, Furusawa C, Hirasawa T, Shimizu H (2012) Stable disruption of ethanol production by deletion of the genes encoding alcohol dehydrogenase isozymes in Saccharomyces cerevisiae. J Biosci Bioeng 113:192–195
Ida Y, Hirasawa T, Furusawa C, Shimizu H (2013) Utilization of Saccharomyces cerevisiae recombinant strain incapable of both ethanol and glycerol biosynthesis for anaerobic bioproduction. Appl Microbiol Biotechnol 97:4811–4819
Yoshikawa K, Hirasawa T, Ogawa K, Hidaka Y, Nakajima T, Furusawa C, Shimizu H (2013) Integrated transcriptomic and metabolomic analysis of the central metabolism of Synechocystis sp. PCC 6803 under different trophic conditions. Biotechnol J 8:571–580
Dettmer K, Aronov PA, Hammock BD (2007) Mass spectrometry-based metabolomics. Mass Spectrom Rev 26:51–78
Soga T, Ohashi Y, Ueno Y, Naraoka H, Tomita M, Nishioka T (2003) Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J Proteome Res 2:488–494
Takagi H, Iwamoto F, Nakamori S (1997) Isolation of freeze-tolerant laboratory strains of Saccharomyces cerevisiae from proline-analogue-resistant mutants. Appl Microbiol Biotechnol 47:405–411
Takagi H, Sakai K, Morida K, Nakamori S (2000) Proline accumulation by mutation or disruption of the proline oxidase gene improves resistance to freezing and desiccation stresses in Saccharomyces cerevisiae. FEMS Microbiol Lett 184:103–108
Takagi H, Takaoka M, Kawaguchi A, Kubo Y (2005) Effect of l-proline on sake brewing and ethanol stress in Saccharomyces cerevisiae. Appl Environ Microbiol 71:8656–8662
Cortes-Rojo C, Calderon-Cortes E, Clemente-Guerrero M, Manzo-Avalos S, Uribe S, Boldogh I, Saavedra-Molina A (2007) Electron transport chain of Saccharomyces cerevisiae mitochondria is inhibited by H2O2 at succinate-cytochrome c oxidoreductase level without lipid peroxidation involvement. Free Radic Res. 41:1212–1223
Acknowledgments
We thank Mr. Tatsuya Itoga for help with the CE-MS measurements. This work was supported by JSPS KAKENHI, Grant-in-Aid for Scientific Research (A) Grant Number 24246134.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nugroho, R.H., Yoshikawa, K., Matsuda, F. et al. Positive effects of proline addition on the central metabolism of wild-type and lactic acid-producing Saccharomyces cerevisiae strains. Bioprocess Biosyst Eng 39, 1711–1716 (2016). https://doi.org/10.1007/s00449-016-1646-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1646-1