Abstract
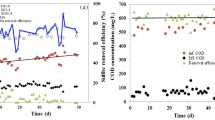
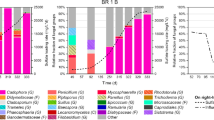
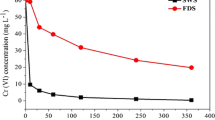
Biological treatment with sulfate-reducing bacteria (SRB) is considered to be an excellent option to remove heavy metals from wastewater. In this study, the optimization of sulfide production for an enhanced removal of lead by a consortium of SRB was carried out based on central composite design and analyzed using response surface methodology (RSM). The sulfide production process was investigated as a function of three independent variables: solution pH (6.5–8.5), lactate concentration (32–96 mM), and sulfate concentration (16–32 mM). RSM analysis showed that the optimum conditions for a high sulfide concentration (14.2 mM) occurred at a pH of 7.5 and at lactate and sulfate concentrations of 53.4 mM and 22.6 mM, respectively. The lead removal efficiency of the SRB consortium using optimum conditions was determined in four parallel anaerobic continuous moving bed biofilm reactors (V = 2 L) that were fed with synthetic wastewater containing dissolved lead at concentrations of 0, 100, 150, 200 mg L−1 and operated with a hydraulic retention time of 5 days. 99–100 % was removed from synthetic wastewater with lead concentrations of 100 and 150 mg L−1 during 40 days of operation. For the highest lead concentration of 200 mg L−1, a decrease in efficiency of removal (96 %) was observed at the end of the experiment.


Similar content being viewed by others
References
Al-Zuhair S, El-Naas MH, Al-Hassani H (2008) Sulfate inhibition effect on sulfate-reducing bacteria. J Biochem Technol 1(2):39–44
American Public Health Association (APHA) (1998) In: Clesceri LS, Greenberg AE, Eaton AD (eds) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association (APHA); Water Environment Federation, Washington, DC
Bahadir T, Bakan G, Aitas L, Buyukgungor H (2007) The investigation of lead removal by biosorption: an application at storage battery industry wastewater. Enzyme Microb Tech 41:98–102
Bhagat M, Burgess JE, Antunes APM, Whiteley CG, Duncan JR (2004) Precipitation of mixed metal residues from wastewater utilizing biogenic sulfide. Min Eng 7:925–932
Bhattacharya SK, Uberoi V, Dronamraju MM (1996) Interaction between acetate fed sulfate reducers and methanogens. Water Res 30(10):2239–2246
Cao J, Zhang G, Mao ZS, Li Y, Fang Z, Yang C (2012) Influence of electron donors on the growth and activity of sulfate-reducing bacteria. Int J Miner Process 106:58–64
Choi E, Rim JM (1991) Competition and inhibition of sulfate reducers and methane producers in anaerobic treatment. Water Sci Technol 23(77):1259–1264
El Bayoumy MA, Bewtra JK, Ali HI, Biswas N (1999) Sulfide production by sulfate reducing bacteria with lactate as feed in an upflow anaerobic fixed film reactor. Water Air Soil Pollut 112(1–2):67–84
Feng Q, Lin Q, Gong F, Sugita S, Shoya M (2004) Adsorption of lead and mecury by rice husk ash. J Colloid Interface Sci 278:1–8
Gallegos-Garcia M, Celis LB, Rangel-Médez R, Razo-Flores E (2009) Precipitation and recovery of metal sulfides from metal containing acidic wastewater in a sulfidogenic down-flow fluidized bed reactor. Biotechnol Bioeng 102:91–99
Hulshof AHM, Blowes DW, Gould WD (2006) Evaluation of in situ layer for treatment of acid mine drainage: a field comparison. Water Res 40:1816–1826
Jong T, Parry DL (2003) Removal of sulfate and heavy metals by sulfate reducing bacteria in short-term bench scale upflow anaerobic packed bed reactor runs. Water Res 37:3379–3389
Kaksonen AH, Franzmann PD, Puhakka JA (2003) Performance and ethanol utilization kinetics of a sulfate-reducing fluidized-bed reactor treating acidic metal-containing wastewater. Biodegradation 14:207–217
Kalyuzhnyi S, Gladchenko M, Epov A, Appanna V (2003) Removal of chemical oxygen demand, nitrogen, and heavy metals using a sequenced anaerobic-aerobic treatment of landfill leachates at 10–30 °C. Appl Biochem Biotechnol 109(1–3):181–195
Kieu TQH, Mueller E, Horn H (2011) Heavy metal removal in anaerobic semi-continuous stirred tank reactor by a consortium of sulfate-reducing bacteria. Water Res 45:3863–3870
Myers H, Montgomery DC (2002) Response surface methodology process and product optimization using designed experiments. Wiley, New York
Neculita CM, Zagury GJ (2007) Bussiere B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: critical review and reasearch needs. J Environ Qual 36:1–16
Oyekola OO, Harrison STL, van Hille RP (2012) Effect of culture conditions on the competitive interaction between lactate oxidizers and fermenters in a biological sulfate reduction system. Biores Technol 104:616–621
Pagnanelli F, Cruz Viggi C, Cibati A, Uccelletti D, Toro L, Palleschi C (2012) Biotreatment of Cr(VI) contaminated wates by sulfate reducing bacteria fed with ethanol. J Hazard Mater 199–200:186–192
Pikuta E, Lysenko A, Suzina N, Osipov G, Kuznetsow B, Tourova T, Akimenkov Laurinavichius K (2000) Desulfotomaculum alkaliphilum sp. Nov., a new alkaliphilic, moderately thermophilic, sulfate-reducing bacterium. Int J Evol Microbiol 50:25–33
Postgate JR (1984) The sulfate-reducing bacteria, 2nd edn. Cambridge University Press, Cambridge
Quan ZX, La HJ, Cho YG, Hwang MH, Kim LS, Lee ST (2003) Treatment of metal-contaminated water and vertical distribution of metal precipitates in an upflow anaerobic bioreactor. Environ Technol 24(3):369–376
Rosen JF (1993) Health effect of lead in children at low exposure levels expert consensus based upon the federal and non-federal literature. In: Allan RJ, Nrigau JO (eds) Metals in the environment, vol II. CEP Consultans, London, p 516
Sani RK, Geesey G, Peyton BM (2001) Assessment of lead toxicity to Desulfovibrio desulfuricans G20: influnce of components of Lactate C medium. Adv Environ Res 5:269–276
Singh R, Kumar A, Kirrolia A, Kumar R, Yadav N, Rajesh Lohchab RK (2001) Removal of sulfate, COD and Cr(VI) in simulated and real wastewater by sulfate reducing bacteria enrichment in small bioreacotr and FTIR study. Biores Technol 101:677–682
Teclu D, Tivchev G, Laing M, Wallis M (2008) Bioremoval of arsenic species from contaminated waters by sulfate-reducing bacteria. Water Res 42:4885–4893
Tsukamoto TK, Killion HA, Miller GC (2004) Column experiments for mifocbiological treatment of acid mine drainage: low-temperature, low-pH and matrix investigation. Water Res 38:1405–1418
Tuppurainen KO, Vaisanen VO (2002) Rintala JA. Zinc removal in anaerobic sulfate-reducing liquid substrate process. Min Eng 15:847–852
Uhrie JL, Drever JI, Colberg PJS, Nesbitt CC (1996) In situ immobilization of heavy metals assocciated with uranium leach mines by bacterial sulfate reduction. Hydrometallurgy 43:231–239
White C, Gadd GM (1996) Mixed sulfate-reducing bacterial cultures for bioprecipitation of toxic metals: factorial and response-surface analysis of the effects of dilution rate, sulfate and substrate concentration. Microbiology 142:2197–2205
Widdel F (1988) Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: Zehnder AJB (ed) Biology of Anaerobic microorganisms. Wiley, New York, pp 469–586
Acknowledgments
The authors acknowledge financial support provided by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106.16-2012.77. We are grateful to Michael Wagner and Stephanie West and other colleagues from Karlsruhe Institute of Technology, Engler-Bunte-Institut, Chair of Water Chemistry and Water Technology (Engler-Bunte-Ring 1, 76131 Karlsruhe, Germany) for giving advice and help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kieu, T.Q.H., Nguyen, T.Y., Dang, T.Y. et al. Optimization of sulfide production by an indigenous consortium of sulfate-reducing bacteria for the treatment of lead-contaminated wastewater. Bioprocess Biosyst Eng 38, 2003–2011 (2015). https://doi.org/10.1007/s00449-015-1441-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1441-4