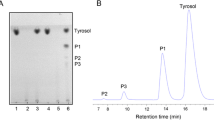
Abstract
We synthesized 2-phenoxyethanol galactoside (PE-Gal) from 2-phenoxyethanol (PE), in which Escherichia coli β-gal (as E. coli cells) and lactose were added in the reaction mixture for galactosylation. About 40 mM PE-Gal was maximally synthesized from about 80 mM PE at 24 h as about 50 % conversion yield. After purifying PE-Gal, the structure of PE-Gal was identified using LC-MS, 1H NMR, and 13C NMR analyses. In addition, it was observed that the water solubility of PE-Gal was increased by galactosylation of PE. The MICs of PE and PE-Gal against Gram-negative and Gram-positive bacteria were fairly similar with each other (23.3–61.3 mM as the average value). PE-Gal was noticeably less cytotoxic against HACAT cells, in particular a remarkable difference in cell viability was observed at concentrations of 20–60 mM PE or PE-Gal. Finally, we accomplished the synthesis of less toxic PE-Gal, compared with PE, using β-gal-containing E. coli cells without changing in the MICs against microorganisms. In the future, PE-Gal will be applicable as a substitute for PE as a less toxic preservative for the cosmetic, pharmaceutical, and food industries.





Similar content being viewed by others
Abbreviations
- CPN:
-
Chlorphenesin
- CPN-Gal:
-
Chlorphenesin galactoside
- E. coli :
-
Escherichia coli
- β-gal:
-
β-Galactosidase
- GOS:
-
Galactooligosaccharide
- IB:
-
Inclusion body
- PE:
-
2-Phenoxyethanol
- PE-Gal:
-
2-Phenoxyethanol galactoside, 2-hydroxyethyl β-d-galactopyranoside
- MIC:
-
Minimum inhibitory concentration
References
Melisi D, Curcio A, Luongo E, Morelli E, Rimoli MG (2011) D-Galactose as a vector for prodrug design. Curr Top Med Chem 11:2288–2298
Davis BG, Robinson MA (2002) Drug delivery systems based on sugar-macromolecule conjugates. Curr Opin Drug Discov Devel 5:279–288
Huang J, Gao F, Tang X, Yu J, Wang D, Liu S, Li Y (2010) Liver-targeting doxorubicin-conjugated polymeric prodrug with pH-triggered drug release profile. Polym Int 59:1390–1396
Tietze LF, Schmuck K (2011) Prodrugs for targeted tumor therapies: recent developments in ADEPT, GDEPT and PMT. Curr Pharm Des 17:3527–3547
Mori T, Fujita S, Okahata Y (1997) Transglycosylation in a two-phase aqueous-organic system with catalysis by a lipid-coated β-D-galactosidase. Carbohydr Res 298:65–73
Vera C, Guerrero C, Illanes A (2011) Determination of the transgalactosylation activity of Aspergillus oryzae β-galactosidase: effect of pH, temperature, and galactose and glucose concentrations. Carbohydr Res 346:745–752
Rodriguez-Colinas B, Fernandez-Arrojo L, de Abreu M, Urrutia P, Fernandez-Lobato M, Ballesteros AO, Plou FJ (2013) On the enzyme specificity for the synthesis of prebiotic galactooligosaccharides. In: Shukla P, Pletschke BI (eds) Advances in enzyme biotechnology. Springer, New York, pp 23–39
Kwon SK, Jung H-C, Pan J-G (2007) Transgalactosylation in a water-solvent biphasic reaction system with β-galactosidase displayed on the surfaces of Bacillus subtilis spores. Appl Environ Microbiol 73:2251–2256
Li C, Kim Y-W (2014) Characterization of a galactosynthase derived from Bacillus circulans β-galactosidase: Facile synthesis of d-Lacto- and d-Galacto-N-bioside. Chem Bio Chem 15:522–526
Badarinath V, Halami PM (2011) Purification of new β-galactosidase from Enterococcus faecium MTCC 5153 with transgalactosylation activity. Food Biotechnol 25:225–239
Lu L, Xu X, Gu G, Jin L, Xiao M, Wang F (2010) Synthesis of novel galactose containing chemicals by β-galactosidase from Enterobacter cloacae B5. Bioresour Technol 101:6868–6872
Liu GX, Kong J, Lu WW, Kong WT, Tian H, Tian XY, Huo GC (2011) β-Galactosidase with transgalactosylation activity from Lactobacillus fermentum K4. J Dairy Sci 94:5811–5820
Schwab C, Lee V, Sørensen KI, Gänzle MG (2011) Production of galactooligosaccharides and heterooligosaccharides with disrupted cell extracts and whole cells of lactic acid bacteria and bifidobacteria. Int Dairy J 21:748–754
Jung K-H (2008) Enhanced enzyme activities of inclusion bodies of recombinant β-galactosidase via the addition of inducer analog after l-arabinose induction in the araBAD promoter system of Escherichia coli. J Microbiol Biotechnol 18:434–442
Jung K-H, Yeon J-H, Moon S-K, Choi JH (2008) Methyl α-d-glucopyranoside enhances the enzymatic activity of recombinant β-galactosidase inclusion bodies in the araBAD promoter system of Escherichia coli. J Ind Microbiol Biotechnol 35:695–701
Lee S-E, Seo H-B, Kim H-J, Yeon J-H, Jung K-H (2011) Galactooligosacchride synthesis by active β-galactosidase inclusion bodies-containing Escherichia coli cells. J Microbiol Biotechnol 21:1151–1158
Zheng P, Yu H, Sun Z, Ni Y, Zhang W, Fan Y, Xu Y (2006) Production of galacto-oligosaccharides by immobilized recombinant β-galactosidase from Aspergillus candidus. Biotechnol J 1:1464–1470
Li Z, Xiao M, Lu L, Li Y (2008) Production of nonmonosaccharide and high-purity galactooligosaccharides by immobilized enzyme catalysis and fermentation with immobilized yeast cells. Process Biochem 43:896–899
Lee S-E, Jo T-M, Lee H-Y, Lee J, Jung K-H (2013) β-Galactosidase-catalyzed synthesis of galactosyl chlorphenesin and its characterization. Appl Biochem Biotechnol 171:1299–1312
Lee S-E, Lee H-Y, Jung K-H (2013) Production of chlorphenesin galactoside by whole cells of β-galactosidase-containing Escherichia coli. J Microbiol Biotechnol 23:826–832
Bridiau N, Taboubi S, Marzouki N, Legoy MD, Maugard T (2006) β-Galactosidase catalyzed selective galactosylation of aromatic compounds. Biotechnol Prog 22:326–330
Scheckermann C, Wagner F, Fischer L (1997) Galactosylation of antibiotics using the β-galactosidase from Aspergillus oryae. Enzyme Microb Technol 20:629–634
Yazar K, Johnsson S, Lind M-L, Boman A, Lidén C (2010) Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermat 64:265–272
Lowe I, Southern J (1994) The antimicrobial activity of phenoxyethanol in vaccines. Lett Appl Microbiol 18:115–116
Shaluei F, Hedayati A, Jahanbakhshi A, Baghfalaki M (2012) Physiological responses of great sturgeon (Huso huso) to different concentrations of 2-phenoxyethanol as an anesthetic. Fish Physiol Biochem 38:1627–1634
Hamilton T, de Gannes GC (2011) Allergic contact dermatitis to preservatives and fragrances in cosmetics. Skin Therapy Lett 16:1–4
Núñez OR, Carballas VC, Carballada GF, Boquete PM (2010) 2-Phenoxyethanol-induced contact urticaria and anaphylaxis. J Investig Allergol Clin Immunol 20:354–355
Hernández B, Ortiz-Frutos FJ, García M, Palencia S, García MC, Iglesias L (2002) Contact urticaria from 2-phenoxyethanol. Contact Dermat 47:44–54
Birnie AJ, English JS (2006) 2-Phenoxyethanol-induced contact urticaria. Contact Dermat 54:349
Musshoff U, Madeja M, Binding N, Witting U, Speckmann E-J (1999) Effects of 2-phenoxyethanol on N-methyl-D-aspartate (NMDA) receptor-mediated ion currents. Arch Toxicol 73:55–59
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175
Yeon J-H, Jung K-H (2010) Operation of packed-bed immobilized cell reactor featuring active β-galactosidase inclusion body-containing recombinant Escherichia coli cells. Biotechnol Bioprocess Eng 15:822–829
Shimizu R, Shimabayashi H, Moriwaki M (2006) Enzymatic production of highly soluble myricitrin glycosides using β-galactosidase. Biosci Biotechnol Biochem 70:940–948
Lundov MD, Johansen JD, Zachariae C, Moesby L (2011) Low-level efficacy of cosmetic preservatives. Int J Cosmet Sci 33:190–196
Fuursted K, Hjort A, Knudsen L (1997) Evaluation of bactericidal activity and lag of regrowth (postantibiotic effect) of five antiseptics on nine bacterial pathogens. J Antimicrob Chemother 40:221–226
Acknowledgments
The research was supported by a grant from the Academic Research Program of Korea National University of Transportation in 2014. The author thanks Ms. Ju-Hye Yeon and Mr. Tae-Min Jo for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Korea National University of Transportation is formerly Chungju National University.
Rights and permissions
About this article
Cite this article
Jung, KH., Lee, HY. Escherichia coli β-galactosidase-catalyzed synthesis of 2-phenoxyethanol galactoside and its characterization. Bioprocess Biosyst Eng 38, 365–372 (2015). https://doi.org/10.1007/s00449-014-1276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1276-4