Abstract
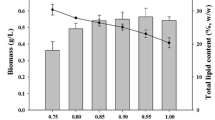
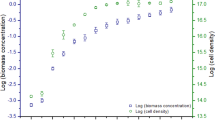
The growth and total lipid content of four green microalgae (Chlorella sp., Chlorella vulgaris CCAP211/11B, Botryococcus braunii FC124 and Scenedesmus obliquus R8) were investigated under different culture conditions. Among the various carbon sources tested, glucose produced the largest biomass or microalgae grown heterotrophically. It was found that 1 % (w/v) glucose was actively utilized by Chlorella sp., C. vulgaris CCAP211/11B and B. braunii FC124, whereas S. obliquus R8 preferred 2 % (w/v) glucose. No significant difference in biomass production was noted between heterotrophic and mixotrophic (heterotrophic with light illumination/exposure) growth conditions, however, less production was observed for autotrophic cultivation. Total lipid content in cells increased by approximately two-fold under mixotrophic cultivation with respect to heterotrophic and autotrophic cultivation. In addition, light intensity had an impact on microalgal growth and total lipid content. The highest total lipid content was observed at 100 μmol m−2s−1 for Chlorella sp. (22.5 %) and S. obliquus R8 (23.7 %) and 80 μmol m−2s−1 for C. vulgaris CCAP211/11B (20.1 %) and B. braunii FC124 (34.9 %).




Similar content being viewed by others
References
Cho S, Ji SC, Hur S, Bae J, Park IS, Song YC (2007) Optimum temperature and salinity conditions for growth of green algae Chlorella ellipsoidea and Nannochloris oculata. Fish Sci 73:1050–1056
Liang YN, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other application: review. Renew Sustain Energy Rev 14:217–232
Yoo C, Jun SY, Lee JY, Ahn CY, Oh HM (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101:S71–S74
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Xu H, Miao XL, Wu QY (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Li XF, Xu H, Wu QY (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771
Cheng Y, Zhou WG, Gao CF, Lan K, Gao Y, Wu QY (2009) Biodiesel production from Jerusalem artichoke (Helianthus Tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides. J Chem Technol Biotechnol 84:777–781
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Huang GH, Chen F, Wei D, Zhang XW, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Hongjin Q, Guangce W (2009) Effect of carbon source on growth and lipid accumulation in Chlorella sorokiniana GXNN01. Chinese J Oceanol Limnol 27:762–768
Leesing R, Nontaso N (2010) Microalgal oil production by green microalgae under heterotrophic cultivation. KKU Res J 15:787–793
Wan M, Liu P, Xia J, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie Z, Qiu G (2011) The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl Microbiol Biotechnol 91:835–844
Ratledge C, Kanagachandran K, Anderson AJ, Grantham DJ, Stephenson JC (2001) Production of docosahexaenoic acid by Crypthecodinium cohnii grown in a pH-auxostat culture with acetic acid as principal carbon source. Lipids 36:12410–12416
Yeh KL, Chang JS, Chen YM (2010) Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng Life Sci 10:201–208
Lee K, Lee C (2002) Nitrogen removal from wastewaters by microalgae without consuming organic carbon sources. J Microbiol Biotechnol 12:979–985
Doan TTY, Sivaloganathan B, Obbard JP (2011) Screening of marine microalgae for biodiesel feedstock. Biomass Bioenergy 35:2534–2544
Yeesang C, Cheirsilp (2011) Effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresour Technol 102:3034–3040
Harris EH (1989) The Chlamydomonas source book: a comprehensive guide to biology and laboratory use. Academic Press, San diego, p 780
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Bold HC (1949) The morphology of Chlamydomonas chlamydogama sp. nov. Bull Torrey Bot Club 76:101–108
Chu SP (1942) The influence of the mineral composition of the medium on the growth of planktonic algae. J Ecol 30(2):284–325
Folch J, Lees M, Stanley GHS (1956) A simple method for isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Droop MR (1974) Heterotrophy of carbon. In: Stewart WDP (ed) Algal physiology biochemistry. Blackwell Scientific Oxford, UK, pp 530–559
Neilson AH, Lewin RA (1974) The uptake and utilization of organic carbon by algae: an essay in comparative biochemistry. Phycologia 13:227–264
Shi XM, Liu HJ, Zhang XW, Chen F (1999) Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process Biochem 34:341–347
Hosoglu MI, Gultepe I, Elibol M (2012) Optimization of carbon and nitrogen sources for biomass and lipid production by Chlorella saccharophila under heterotrophic conditions and development of Nile red fluorescence based method for quantification of its neutral lipid content. Biochem Eng J 61:11–19
Tan CK, Johns MR (1991) Fatty acid production by heterotrophic Chlorella saccharophila. Hydrobiologia 215:13–19
Yang C, Hua Q, Shimizu K (2000) Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J 6:87–102
Yokota A, Harada A, Kitaoka S (1989) Characterization of ribulose 1,5-bisphosphate carboxylase/oxygenase from Euglena gracilis Z. J Biochem 105:400–405
Arroyo TH, Wei W, Ruan R, Hu B (2011) Mixotrophic cultivation of Chlorella vulgaris and its potential application for oil accumulation from non-sugar materials. Biomass Bioenergy 35:2245–2253
Khotimchenko SV, Yakovleva IM (2005) Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phytochemistry 66:73–79
Tansakul P, Savaddirakasa Y, Prasertsan P, Tongurai C (2005) Cultivation of the hydrocarbon-rich alga, Botryococcus braunii in secondary treated effluent from a sea food processing plant. Thai J Agric Sci 38:71–76
Sauer A, Heise KP (1984) Regulation of acetyl-coenzyme A carboxylase and acetyl-coenzyme A synthetase in spinach chloroplasts. Z Naturforschung 39:268–275
Guihéneuf F, Mimouni V, Ulmann L, Tremblin G (2009) Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalgae pavlova lutheri commonly used in mariculture. J Exp Marine Biol Ecol 369:136–143
Mock T, Kroon BMA (2002) Photosynthetic energy conversion under extreme conditions-II: the significance of lipids under light limited growth in antractic sea ice diatoms. Phytochemistry 61:53–60
Sukenik A, Carmell Y, Berner T (1989) Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J Phycol 25:686–692
Rammus J (1990) A form-function analysis of photon captures for seaweeds. Hydrobiologia 204–205:65–71
Ho SH, Lu WB, Chang JS (2012) Photobioreactor strategies for improving the CO2 fixation effiency of indigenous Scenedesmus obliquus CNW-N: statistical optimization of CO2 feeding. Bioresour Technol 105:106–113
Morais MG, Costa JAV (2007) Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol Lett 29:1349–1352
Pal D, Goldberg IK, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Gordillo FJL, Goutx M, Figueroa FL, Niell FX (1998) Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J Appl Phycol 10:135–144
Kim W, Park JM, Gim GH, Jeong S, Kang CM, Kim D, Kim SW (2012) Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst Eng 35:19–27
Chen YH, Walker TH (2011) Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol Lett 33:1973–1983
Sydney EB, Sturm W, Carvalho JC, Soccol VT, Larroche C, Pandey A, Soccol CR (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 10:5892–5896
Acknowledgments
This work was supported by the grant of New & Renewable Energy Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) funded by the Ministry of Knowledge Economy, Korea (20103020090020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gim, G.H., Kim, J.K., Kim, H.S. et al. Comparison of biomass production and total lipid content of freshwater green microalgae cultivated under various culture conditions. Bioprocess Biosyst Eng 37, 99–106 (2014). https://doi.org/10.1007/s00449-013-0920-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-0920-8