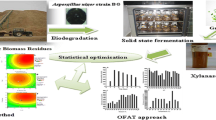
Abstract
This study aimed to assess the variability in respect of titer and properties of xylanase from Trichoderma reesei SAF3 under both solid-state and submerged fermentation. SSF was initially optimized with different agro-residues and among them wheat bran was found to be the best substrate that favored maximum xylanase production of 219 U (gws)−1 at 96 h of incubation. The mycelial stage of the fungi and intracellular accumulation of Ca++ and Mg++ induced maximum enzyme synthesis. Inoculum level of 10 × 106 spores 5 g−1 of dry solid substrate and water activity of 0.6 were found to be optimum for xylanase production under SSF. Further optimization was made using a Box-Behnken design under response surface methodology. The optimal cultivation conditions predicted from canonical analysis of this model were incubation time (A) = 96–99 h, inoculum concentration (B) = 10 × 106 spores 5 g−1 of dry substrate, solid substrate concentration (C) = 10–12 g flask−1, initial moisture level (D) = 10 mL flask−1 (equivalent to a w = 0.55) and the level of xylanase was 299.7 U (gws)−1. Subsequent verification of these levels agreed (97 % similar) with model predictions. Maximum amount of xylanase was recovered with water (6:1, v/w) and under shaking condition (125 rpm). Purified xylanase from SSF showed better stability in salt and pH, was catalytically and thermodynamically more efficient over enzyme from SmF, though molecular weight of both enzymes was identical (53.8 kDa).






Similar content being viewed by others
References
Pandey A, Soccol CR, Mitchell D (2000) New developments in solid state fermentation: I. Bioprocesses and products. Proc Biochem 35:1153–1169
Someet N, Virendra S (2001) Optimization of xylanase production by Melanocarpus albomyces IIS 68 in solid-state fermentation using response surface methodology. J Biosci Bioeng 91:425–427
Holker U, Hofer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
Viniegra-González G, Favela-Torres E, Aguilar CN, Rómero-Gomez SJ, D′ıaz-God′ınez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Lekha P, Lonsane B (1994) Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL 104 in solid-state, liquid surface and submerged fermentations. Proc Biochem 29:497–503
Acuna-Arguelles ME, Gutierrez-Rojas M, Viniegra-González G, Favela-Torres E (1995) Production and properties of three pectinolytic activities produced by Aspergillus niger in submerged and solid-state fermentation. Appl Microbiol Biotechnol 43:808–814
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation for the production of industrial enzyme. Curr Sci 77:149–162
Krishna C (2005) Solid-state fermentation systems—an overview. Crit Rev Biotechnol 25:1–30
Manimaran A, Kumar KS, Permaul K, Singh S (2009) Hyper production of cellulase-free xylanase by Thermomyces lanuginosus SSBP on bagasse pulp and its application in biobleaching. Appl Microbiol Biotechnol 81:887–893
Li K, Azadi P, Collins R, Tolan J, Kim JS, Eriksson KEL (2000) Relationship between activities of xylanases and xylan structure. Enzym Microbiol Technol 193:265–275
Nagar S, Gupta VK, Kumar D, Kumar L, Kuhad RC (2010) Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. J Indust Microbiol Biotechnol 37:71–83
Miller GL (1959) Developed DNS based method for reducing sugar estimation. Anal Chem 31:246–248
Rashid MH, Siddiqui KS (1998) Thermodynamic and kinetic study of stability of the native and chemically modified β-glucosidase from Aspergillus niger. Proc Biochem 33:109–115
Gawande PV, Kamat MY (1999) Production of Aspergillus xylanase by lignocellulosic waste fermentation and its application. J Appl Microbiol 87:511–519
Okafor UA, Okochi VI, Onyegeme-okerenta BM, Nwodo-Chinedu S (2007) Xylanase production by Aspergillus niger ANL 301 using agro-wastes. Afr J Biotechnol 6:1710–1714
Mandal A, Kar S, Das Mohapatra PK, Maity C, Pati BR, Mondal KC (2012) Regulation of xylanase biosynthesis in Bacillus cereus BSA1. Appl Biochem Biotechnol. doi:10.1007/s12010-011-9523-5
Sun X, Liu Z, Qu Y, Li X (2008) The Effects of wheat bran composition on the production of biomass-hydrolyzing enzymes by Penicillium decumbens. Appl Biochem Biotechnol 146:119–128
Colina A, Sulbaran DFB, Aiello C, Ferrer A (2003) Xylanase production by Trichoderma reesei rut C-30 on rice straw. Appl Biochem Biotechnol 108:715–724
Kar S, Mandal A, Das Mohapatra PK, Mondal KC, Pati BR (2006) Production of cellulase- free xylanase by Trichoderma reesei SAF3. Br J Microbiol 37:462–464
Kar S, Mandal A, Das Mohapatra PK, Samanta S, Pati BR, Mondal KC (2008) Production of xylanase by immobilized Trichoderma reesei SAF3 in Ca-alginate beads. J Ind Microbiol Biotechnol 35:245–249
Park YK, Rivera BC (1982) Alcohol production from various enzyme converted starches with or without cooking. Biotechnol Bioeng 27:259–273
Kamra P, Satyanarayan T (2004) Xylanase production by the thermophilic mold Humicola lanuginosa in solid-state fermentation. Appl Biochem Biotechnol 119:145–158
Xiong H, Von WN, Turunen O, Leisola M, Pastinen O (2005) Xylanase production by Trichoderma reesei Rut C-30 grown on l-arabinose-rich plant hydrolysates. Biores Technol 96:753–759
Isil S, Nilufer A (2005) Investigation of factors affecting xylanase activity from Trichoderma harzianum 1073 D3. Br Arch Biol Technol 48:187–193
Raimbault M, Alazad D (1980) Culture method to study fungal growth in solid fermentation. Eur J Appl Microbiol Biotechnol 9:199–209
Pandey A (1992) Recent process development in solid-state fermentation. Proc Biochem 27:109–117
Lu W, Li D, Wu Y (2003) Influence of water activity and temperature on xylanase biosynthesis in pilot-scale solid-state fermentation by Aspergillus sulphureus. Enzym Microbiol Technol 32:305–311
Mahalaxmi Y, Sathish T, Rao CS, Prakasham RS (2010) Corn husk as a novel substrate for the production of rifamycin B by isolated Amycolatopsis sp. RSP 3 under SSF. Proc Biochem 45:47–53
Nutan D, Ulka SP, Kulbhusan BB, Jayant MK, Digamber VG (2002) Production of acidic lipase by Aspergillus niger in solid-state fermentation. Proc Biochem 38:715–721
Batan B, Sharma J, Kuhud R (2006) High level xylanase production by alkalophilic Bacillus pumilus ASH under solid-state fermentation. World J Microbiol Biotechnol 22:1281–1287
Madeira JV Jr, Macedo JA, Macedo GA (2012) A new process for simultaneous production of tannase and phytase by Paecilomyces variotii in solid-state fermentation of orange pomace. Bioprocess Biosyst Eng 35:477–482
Maity C, Ghosh K, Halder SK, Jana A, Adak A, Das Mohapatra PK, Pati BR, Mondal KC (2012) Xylanase isozymes from the newly isolated Bacillus sp. CKBx1D and optimization of its deinking potentiality. Appl Biochem Biotechnol. doi:10.1007/s12010-012-9556-4
Mandal A, Kar S, Das Mohapatra PK, Maity C, Pati BR, Mondal KC (2011) Purification and characterization of an endoxylanase from the culture broth of Bacillus cereus BSA11. Appl Biochem Microbiol 47:250–255
Jana M, Pati B (1997) Thermostable, salt-tolerant α-amylase from Bacillus sp. MD 124. J Basic Microbiol 37:323–326
Siddiqui KS, Rashid MH, Rajoka MI (1997) Kinetic analysis of the active-site of an intracellular β-glucosidase from Cellulomonas biazotea. Folia Microbiol 42:53–58
Rajoka MI, Akhtar MW, Hanif A, Khalid AM (2006) Production and characterization of a highly active cellobiase from Aspergillus niger grown in solid state fermentation. World J Microbiol Biotechnol 22:991–998
Acknowledgments
The authors are greatly indebted to the University Grants Commission, New Delhi (F.PSW-060/70-08) and CSIR, New Delhi [38 (1234)/09/EMR-II] for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kar, S., Sona Gauri, S., Das, A. et al. Process optimization of xylanase production using cheap solid substrate by Trichoderma reesei SAF3 and study on the alteration of behavioral properties of enzyme obtained from SSF and SmF. Bioprocess Biosyst Eng 36, 57–68 (2013). https://doi.org/10.1007/s00449-012-0761-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-012-0761-x