Abstract
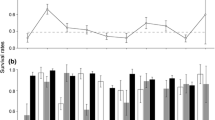
The range of a species is determined by the balance of its demographic rates across space. Population growth rates are widely hypothesized to be greatest at the geographic center of the species range, but indirect empirical support for this pattern using abundance as a proxy has been mixed, and demographic rates are rarely quantified on a large spatial scale. Therefore, the texture of how demographic rates of a species vary over its range remains an open question. We quantified seasonal fecundity of populations spanning the majority of the global range of a single species, the saltmarsh sparrow (Ammodramus caudacutus), which demonstrates a peak of abundance at the geographic center of its range. We used a novel, population projection method to estimate seasonal fecundity inclusive of seasonal and spatial variation in life history traits that contribute to seasonal fecundity. We replicated our study over 3 years, and compared seasonal fecundity to latitude and distance among plots. We observed large-scale patterns in some life history traits that contribute to seasonal fecundity, such as an increase in clutch size with latitude. However, we observed no relationship between latitude and seasonal fecundity. Instead, fecundity varied greatly among plots separated by as little as 1 km. Our results do not support the hypothesis that demographic rates are highest at the geographic and abundance center of a species range, but rather they suggest that local drivers strongly influence saltmarsh sparrow fecundity across their global range.




Similar content being viewed by others
References
Bayard TS, Elphick CS (2010) Using spatial point-pattern assessment to understand the social and environmental mechanisms that drive avian habitat selection. Auk 127:485–494. doi:10.1525/auk.2010.09089
Bayard TS, Elphick CS (2011) Planning for sea-level rise: quantifying patterns of saltmarsh sparrow (Ammodramus caudacutus) nest flooding under current sea-level conditions. Auk 128:393–403. doi:10.1525/auk.2011.10178
Bennett RS, Etterson MA (2007) Incorporating results of avian toxicity tests into a model of annual reproductive success. Integr Environ Assess Manag 3:498–507
Blackburn TM, Gaston KJ, Quinn RM, Gregory RD (1999) Do local abundances of British birds change with proximity to range edge? J Biogeogr 26:493–505. doi:10.1046/j.1365-2699.1999.00298.x
Bradford MJ, Taylor GC, Allan JA (1997) Empirical review of coho salmon smolt abundance and the prediction of smolt production at the regional level. Trans Am Fish Soc 126:49–64. doi:10.1577/1548-8659(1997)126
Brewer AM, Gaston KJ (2003) The geographical range structure of the holly leaf-miner. II. Demographic rates. J Anim Ecol 72:82–93. doi:10.1046/j.1365-2656.2003.00682.x
Brown JH (1984) On the relationship between abundance and distribution of species. Am Soc Nat 124:255–279. doi:10.1086/284267
Brown JH (1995) Macroecology. University of Chicago Press, Chicago
Brown JH, Mehlman DW, Stevens GC (1997) Spatial variation in abundance. Ecology 76:2028–2043
Brussard PF (1984) Geographic patterns and environmental gradients: the central-marginal model in Drosophila revisited. Annu Rev Ecol Syst 15:25–64. doi:10.1146/annurev.es.15.110184.000325
Emlen JT, Dejong MJ, Jaeger MJ et al (1986) Density trends and range boundary constraints of forest birds along a latitudinal gradient. Auk 103:791–803
Enquist BJ, Jordan MA, Brown JH (1995) Connections between ecology biogeography and paleobiology: relationship between local abundance and geographic-distribution in fossil and recent molluscs. Evol Ecol 9:586–604. doi:10.1007/BF01237657
Erwin RM, Sanders GM, Prosser DJ, et al (2006) High tides and rising seas: potential effects on estuarine waterbirds. Terr Vertebr tidal marshes. Evol Ecol Conserv:214–228
Etterson MA, Bennett RS (2013) Quantifying the effects of pesticide exposure on annual reproductive success of birds. Integr Environ Assess Manag 9:590–599. doi:10.1002/ieam.1450
Field CR (2016) A threatened ecosystem in a human-dominated landscape: tidal marsh conservation in the face of sea-level rise. Ph.D. Dissertation. University of Connecticut, Storrs, CT
Gaston KJ (2003) The structure and dynamics of geographic ranges. Oxford University Press, New York
Gaston KJ (2009) Geographic range limits: achieving synthesis. Proc R Soc B Biol Sci 276:1395–1406. doi:10.1098/rspb.2008.1480
Ghalambor CK, Martin TE (2001) Fecundity-survival trade-offs and parental risk-taking in birds. Science 292(80):494–497. doi:10.1126/science.1059379
Gibbons DW, Reid JB, Chapman RA (1993) A new atlas of breeding birds in Britain and Ireland, 1988–1991. Academic Press, London
Gjerdrum C, Elphick CS, Rubega M (2005) Nest site selection and nesting success in saltmarsh breeding sparrows: the importance of nest habitat, timing, and study site differences. Condor 107:849–862
Gjerdrum C, Sullivan-Wiley K, King E (2008) Egg and chick fates during tidal flooding of Saltmarsh Sharp-tailed Sparrow nests. Condor 110:579–584. doi:10.1525/cond.2008.8559
Greenlaw JS, Rising JD (1994) Saltmarsh Sparrow (Ammodramus caudacutus). Birds N Am Online. doi:10.2173/bna.112
Grinnell J (1904) The origin and distribution of the Chest-Nut-Backed Chickadee. Auk 21:364–382
Guo Q, Taper M, Schoenberger M, Brandle J (2005) Spatial-temporal population dynamics across species range: from centre to margin. Oikos 108:47–57
Haldane JBS (1956) The relation between density regulation and natural selection. Proc R Soc B Biol Sci 145:306–308. doi:10.1098/rspb.1979.0086
Hengeveld R, Haeck J (1982) The distribution of abundance. J Biogeogr 9:303–316
Hodgman TP, Shriver WG, Vickery PD (2002) Redefining range overlap between the sharp-tailed sparrows of coastal New England. Wilson Bull 114:38–43. doi:10.1676/0043-5643(2002)114[0038:RROBTS]2.0.CO;2
Hodgman TP, Elphick CS, Olsen BJ et al (2015) The conservation of tidal marsh birds: guiding action at the intersection of our changing land and seascapes
Jackson WB (1965) Litter size in relation to latitude in two murid rodents. Am Midl Nat 73:245–247
Kluth C, Bruelheide H (2005) Effects of range position, inter-annual variation and density on demographic transition rates of Hornungia petraea populations. Oecologia 145:382–393. doi:10.1007/s00442-005-0141-1
Lack D (1947) Significance of clutch size. Ibis (Lond 1859) 89:302–352
Lawton J (1993) Range, population abundance and conservation. 8:409–413
Leisnham PT, Sala LM, Juliano SA (2008) Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. J Med Entomol 45:210–221. doi:10.1093/jmedent/45.2.210
Litzgus JD, Mousseau TA (2003) Multiple clutching in southern spotted turtles, Clemmys guttata. J Herpetol 37:17–23. doi:10.1670/0022-1511(2003)037
Lord RD (1960) Litter size and latitude in North American mammals. Am Midl Nat 64:488–499
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Martin TE (1996) Life history evolution in tropical and south temperate birds: what do we really know? J Avian Biol 27:263–272. doi:10.2307/3677257
Oksanen AJ, Blanchet FG, Kindt R et al (2016) Package “vegan”
Olsen BJ, Felch JM, Greenberg R, Walters JR (2008) Causes of reduced clutch size in a tidal marsh endemic. Oecologia 158:421–435. doi:10.1007/s00442-008-1148-1
Pianka ER (1970) Comparative autecology of the lizard Cnemidophorus tigris in different pards of its geographic range. Ecology 51:703–720. doi:10.2307/1934053
Pulliam H (1988) Sources, sinks, and population regulation. Am Nat 132:652–661
Purves DW (2009) The demography of range boundaries versus range cores in eastern US tree species. Proc R Soc B Biol Sci 276:1477–1484. doi:10.1098/rspb.2008.1241
R Core Team (2014) R: a language and environment for statistical computing
Rapoport EH (1982) Areography. Pergamon Press, New York
Rhainds M, Fagan WF (2010) Broad-scale latitudinal variation in female reproductive success contributes to the maintenance of a geographic range boundary in bagworms (Lepidoptera: Psychidae). PLoS One 5:e14166. doi:10.1371/journal.pone.0014166
Ripley B, Venables W (2015) Package “nnet”. https://cran.r-project.org/web/packages/nnet/index.html
Rogers DJ, Randolph SE (1986) Distribution and abundance of Tsetse flies (Glossina Spp.). J Anim Ecol 55:1007–1025
Root T (1988) Atlas of wintering North American birds: an analysis of Christmas Bird Count data. University of Chicago Press, Chicago
Rosenberg KV, Pashley D, Andres B et al (2014) The state of the birds 2014 watch list. Washington, D.C
Saetre GP, Borge T, Lindell J et al (2001) Speciation, introgressive hybridization and nonlinear rate of molecular evolution in flycatchers. Mol Ecol 10:737–749
Sagarin R, Gaines S (2002a) The “abundant centre”distribution: to what extent is it a biogeographical rule? Ecol Lett 5:137–147. doi:10.1046/j.1461-0248.2002.00297.x
Sagarin RD, Gaines SD (2002b) Geographical abundance distributions of coastal invertebrates: using one-dimensional ranges to test biogeographic hypotheses. J Biogeogr 29:985–997. doi:10.1046/j.1365-2699.2002.00705.x
Sagarin RD, Gaines SD, Gaylord B (2006) Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol Evol 21:524–530. doi:10.1016/j.tree.2006.06.008
Samis KE, Eckert CG (2007) Testing the abundant center model using range-wide demographic surveys of two coastal dune plants. Ecology 88:1747–1758. doi:10.2307/27651292
Scott JM, Mountainspring S, Ramsey FL, Kepler CB (1986) Forest bird communities of the Hawaiian Islands: their dynamics, ecology, and conservation. Stud Avian Biol 9:1–431
Shriver WG, Vickery PD, Hodgman TP (2007) Flood tides affect breeding ecology of two sympatric sharp-tailed sparrows. Auk 124:552–560. doi:10.1642/0004-8038(2007)124[552:FTABEO]2.0.CO;2
Svensson BW (1992) Changes in occupancy, niche breadth and abundance of three Gyrinus species as their respective range limits are approached. Oikos 63:147–156. doi:10.2307/3545524
Tiner RW (2013) Tidal wetlands primer: an introduction to their ecology, natural history, status, and conservation. University of Massachusetts Press, Amherst
Walsh J, Kovach AI, Lane OP et al (2011) Genetic barcode RFLP analysis of the Nelson’s and Saltmarsh Sparrow hybrid zone. Wilson J Ornithol 123:316–322. doi:10.1676/10-134.1
Walsh J, Kovach A, Babbitt K, O’Brien K (2012) Fine-scale population structure and asymmetrical dispersal in an obligate salt-marsh passerine, the Saltmarsh Sparrow (Ammodramus caudacutus). Auk 129:247–258. doi:10.1525/auk.2012.11153
Walsh J, Rowe RJ, Olsen BJ et al (2015a) Genotype-environment associations support a mosaic hybrid zone between two tidal marsh birds. Ecol Evol 2:279–294. doi:10.1002/ece3.1864
Walsh J, Shriver WG, Olsen BJ et al (2015b) Relationship of phenotypic variation and genetic admixture in the Saltmarsh–Nelson’s sparrow hybrid zone. Auk 132:704–716. doi:10.1642/AUK-14-299.1
Wiens JA (1989) The ecology of bird communities, volume 1 foundations and patterns. Cambridge University Press, Cambridge
Wiest WA, Correll MD, Olsen BJ et al (2016) Population estimates for tidal marsh birds of high conservation concern in the northeastern USA from a design-based survey. Condor 118:274–288. doi:10.1650/CONDOR-15-30.1
Acknowledgments
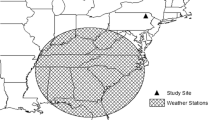
This work was primarily funded by a Competitive State Wildlife Grant (U2-5-R-1) via the United States Fish and Wildlife Service, Federal Aid in Sportfish and Wildlife Restoration to the states of Delaware, Maryland, Connecticut, and Maine. We received additional funding from the United States Fish and Wildlife Service (Region 5, Division of Natural Resources, National Wildlife Refuge System), the United States Department of Agriculture (National Institute of Food and Agriculture NH McIntire-Stennis Project 225,575), the New York Department of Environmental Conservation (AM08634), and the National Science Foundation (DEB-1340008). This project was supported by the USDA National Institute of Food and Agriculture, project number #ME0-H-6-00492-12. This is Maine Agricultural and Forest Experiment Station Publication Number 3501. Graduate students were also funded in part by the National Science Foundation, the National Park Service Gateway Learning Center Fellowship, the University of Maine, the University of New Hampshire, and the University of Connecticut. Thank you to our many collaborators, land owners who allowed access to the plots, and to the dozens of field technicians who helped to collect these data. We also thank BJ McGill for his help at various stages of writing this manuscript and MD Correll for the use of her map. Appropriate animal care was ensured by the Institutional Animal Care and Use Committee of the University of Maine under approval A2011-04-02, University of New Hampshire under approvals 100605 and 130604, State University of New York College of Environmental Science and Forestry under approval 120101, University of Connecticut under approval A11-013, and the University of Delaware under approval AUP1157-2015-2. The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the USFWS or any other funding agency.
Author contribution statement
KJR wrote the paper with comments from all authors, particularly MAE, TPH, and BJO; MAE developed the models MCestimate and MCnest that were used in this analysis; all authors participated in data collection; BJO, TPH, CSE, WGS, AIK, and JBC obtained funding for this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hannu J. Ylonen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruskin, K.J., Etterson, M.A., Hodgman, T.P. et al. Seasonal fecundity is not related to geographic position across a species’ global range despite a central peak in abundance. Oecologia 183, 291–301 (2017). https://doi.org/10.1007/s00442-016-3745-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3745-8