Abstract
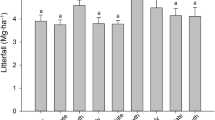
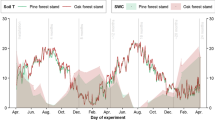
We investigated how forest composition, litter quality, and rainfall interact to affect leaf litter decomposition across three successional tropical dry forests in Costa Rica. We monitored litter stocks and bulk litter turnover in 18 plots that exhibit substantial variation in soil characteristics, tree community structure, fungal communities (including forests dominated by ecto- or arbuscular mycorrhizal host trees), and forest age. Simultaneously, we decomposed three standard litter substrates over a 6-month period spanning an unusually intense drought. Decay rates of standard substrates depended on the interaction between litter identity and forest type. Decomposition rates were correlated with tree and soil fungal community composition as well as soil fertility, but these relationships differed among litter types. In low fertility soils dominated by ectomycorrhizal oak trees, bulk litter turnover rates were low, regardless of soil moisture. By contrast, in higher fertility soils that supported mostly arbuscular mycorrhizal trees, bulk litter decay rates were strongly dependent on seasonal water availability. Both measures of decomposition increased with forest age, as did the frequency of termite-mediated wood decay. Taken together, our results demonstrate that soils and forest age exert strong control over decomposition dynamics in these tropical dry forests, either directly through effects on microclimate and nutrients, or indirectly by affecting tree and microbial community composition and traits, such as litter quality.




Similar content being viewed by others
References
Anaya CA, Garcia-Oliva F, Jaramillo VJ (2007) Rainfall and labile carbon availability control litter nitrogen dynamics in a tropical dry forest. Oecologia 150:602–610
Anaya CA, Jaramillo VJ, Martinez-Yrizar A, Garcia-Oliva F (2012) Large rainfall pulses control litter decomposition in a tropical dry forest: evidence from an 8-year study. Ecosystems 15:652–663
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545
Ayres E, Steltzer H, Berg S, Wall DH (2009) Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. J Ecol 97:901–912
Becknell JM, Powers JS (2014) Stand age and soils as drivers of plant functional traits and aboveground biomass in secondary tropical dry forest. Can J For Res 44:603–614
Burke IC, Kaye JP, Bird SP, Hall SA, McCulley RL, Sommerville GL (2003) Evaluating and testing models of terrestrial biogeochemistry: the role of temperature in controlling decomposition. In: Canham CD, Cole JJ, Lauenroth WK (eds) Models in ecosystem science. Princeton University Press, Princeton, pp 225–253
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaa E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, vanBodegom P, Brovkin V, Chatain A, Callaghan TV, Dıaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E (2013) The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995
Coûteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Ewel JJ (1976) Litter fall and leaf decomposition in a tropical forest succession in Eastern Guatemala. J Ecol 64:293–308
Fernandez CW, Kennedy PG (2015) Revisiting the ‘Gadgil effect’: do inter-guild fungal interactions drive carbon cycling in forest soils? New Phytol. doi:10.1111/nph.13648
Freschet GT, Aerts R, Cornelissen JHC (2012) A plant economics spectrum of litter decomposability. Funct Ecol 26:56–65
Gadgil R, Gadgil PD (1971) Mycorrhiza and litter decomposition. Nature 233:133
Gutiérrez-Leitón M (2013) Déficit hídrico en cifras. Estación Experimental Forestal Horizontes, Liberia, pp 1–3
Hatfield R, Fukushima RS (2005) Can lignin be accurately measured? Crop Sci 45:832–839
Hattenschwiler S, Jorgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Jiménez-Rodríguez CD, Calvo-Alvarado J (2014) An evaluation of rainfall interception in secondary tropical dry forests. In: Sanchez-Azofeifa GA, Powers JS (eds) Tropical dry forests in the americas: ecology, conservation, and management. CRC Press, Boca Raton, pp 249–265
Kaffenberger JT, Schilling JS (2015) Comparing lignocellulose physiochemistry after decomposition by brown rot fungi with distinct evolutionary origins. Environ Microbiol. doi:10.1111/1462-2920.12615
Keiser AD, Knoepp JD, Bradford MA (2013) Microbial communities may modify how litter quality affects potential decomposition rates as tree species migrate. Plant Soil 372:167–176
Kissing LB, Powers JS (2010) Coarse woody debris stocks as a function of forest type and stand age in Costa Rican tropical dry forest: long-lasting legacies of previous land use. J Trop Ecol 26:467–471
Koide RT, Wu T (2003) Ectomycorrhizas and retarded decomposition in a Pinus resinosa plantation. New Phytol 158:401–407
Lebrija-Trejos E, Perez-Garcia EA, Meave JA, Poorter L, Bongers F (2011) Environmental changes during secondary succession in a tropical dry forest in Mexico. J Trop Ecol 27:477–489
Lindahl BD, Tunlid A (2015) Ectomycorrhizal fungi—potential organic matter decomposers, yet not saprotrophs. New Phytol 205:1443–1447
Lohbeck M, Poorter L, Martinez-Ramos M, Bongers F (2015) Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 96:1242–1252
Makkonen M, Berg MP, Handa IT, Hattenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041
Maynard DS, Crowther TW, King JR, Warren RJ, Bradford MA (2015) Temperate forest termites: ecology, biogeography, and ecosystem impacts. Ecological Entomology 40(3):199–210. doi:10.1111/een.12185
McGuire KL, Treseder KK (2010) Microbial communities and their relevance for ecosystem models: decomposition as a case study. Soil Biol Biochem 42:529–535
McGuire KL, Zak DR, Edwards IP, Blackwood CB, Upchurch R (2010) Slowed decomposition is biotically mediated in an ectomycorrhizal, tropical rain forest. Oecologia 164:785–795
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Midgley MG, Brzostek E, Phillips RP (2015) Decay rates of leaf litters from arbuscular mycorrhizal trees are more sensitive to soil effects than litters from ectomycorrhizal trees. J Ecol 103:1454–1463
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Ostertag R, Marin-Spiotta E, Silver WL, Schulten J (2008) Litterfall and decomposition in relation to soil carbon pools along a secondary forest chronosequence in Puerto Rico. Ecosystems 11:701–714
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy, a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51
Powers JS, Perez-Aviles D (2013) Edaphic factors are a more important control on surface fine roots than stand age in secondary tropical dry forests. Biotropica 45:1–9
Powers JS, Montgomery RA, Adair EC, Brearley FQ, DeWalt SJ, Castanho CT, Chave J, Deinert E, Ganzhorn JU, Gilbert ME, Antonio-Gonzalez J, Bunyavejchewin S, Grau HR, Harms KE, Hiremath A, Iriarte-Vivar S, Manzane E, Oliveira AAd, Poorter L, Ramanamanjato J-B, Salk C, Varela A, Weiblen GD, Lerdau MT (2009a) Decomposition in tropical forests: a pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion. J Ecol 97:801–811
Powers JS, Becknell JM, Irving J, Perez-Aviles D (2009b) Diversity and structure of regenerating tropical dry forests in Costa Rica, geographic patterns and environmental drivers. For Ecol Manage 258:959–970
R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0, URL http://www.R-project.org
Schilling JS, Ayres A, Kaffenberger JT, Powers JS (2015) Initial white rot type dominance of wood decomposition and its functional consequences in a regenerating tropical dry forest. Soil Biol Biochem 88:58–68
Sluiter J, Sluiter A (2010) Summative mass closure (NREL/TP-510-48087). National Renewable Energy Laboratory, Golden
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass (NREL.TP-510-42618). National Renewable Energy Laboratory, Golden
Soto M (2014) Fuerte sequía en Guanacaste adelantaría incendios forestales. La Nación, 13 September 2014
Strickland MS, Lauber C, Fierer N, Bradford MA (2009) Testing the functional significance of microbial community composition. Ecology 90:441–451
Strickland MS, Keiser AD, Bradford MA (2015) Climate history shapes contemporary leaf litter decomposition. Biogeochemistry 122:165–174
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 93:345–354
Talbot JM, Allion SD, Treseder KK (2008) Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–963
Ulate GV (2001) Fitogeografía de ecosistemas secos el la meseta de ignimbritas de Guanacaste, Costa Rica. Rev Biol Trop 49:227–238
Veen GF, Freschet GT, Ordonez A, Wardle DA (2015) Litter quality and environmental controls of home-field advantage effects on leaf litter decomposition. Oikos 124:187–195
Waring BG (2012) A meta-analysis of climatic and chemical controls on leaf litter decay rates in tropical forests. Ecosystems 15:999–1009
Waring BG, Becknell JM, Powers JS (2015) Nitrogen, phosphorus, and cation use efficiency in stands of regenerating tropical dry forest. Oecologia 178:887–897
Waring BG, Adams R, Branco S, Powers JS (2016) Scale-dependent variation in nitrogen cycling and soil fungal communities along gradients of forest composition and age in regenerating tropical dry forests. New Phytol 209:845–854
Wieder RK, Wright SJ (1995) Tropical forest litter dynamics and dry season irrigation on Barro Colorado Island, Panama. Ecology 76:1971–1979
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90:3333–3341
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93
Acknowledgments
We gratefully acknowledge financial support from the United States National Science Foundation (Career grant DEB 1053237 to J.S.P), excellent help in the field from Daniel Peréz, and logistical support from Roger Blanco and Maria Marta Chavarría and the Área de Conservación Guanacaste.
Author contribution statement
JSP and BGW conceived and designed the study. EMS and BGW performed the field research, and JSS performed lab analysis. BGW and JSP analyzed the data and wrote the paper with input from EMS and JSS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Russell K. Monson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schilling, E.M., Waring, B.G., Schilling, J.S. et al. Forest composition modifies litter dynamics and decomposition in regenerating tropical dry forest. Oecologia 182, 287–297 (2016). https://doi.org/10.1007/s00442-016-3662-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-016-3662-x