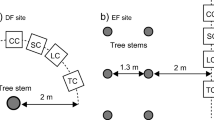
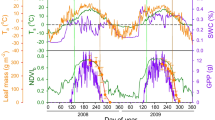
Abstract
The functional equilibrium between roots and shoots suggests an intrinsic linkage between belowground and aboveground phenology. However, much less understanding of belowground phenology hinders integrating belowground and aboveground phenology. We measured root respiration (R a) as a surrogate for root phenology and integrated it with observed leaf phenology and radial growth in a birch (Betula platyphylla)–aspen (Populus davidiana) forest and an adjacent larch (Larix gmelinii) forest in Northeast China. A log-normal model successfully described the seasonal variations of R a and indicated the initiation, termination and peak date of root phenology. Both root phenology and leaf phenology were highly specific, with a later onset, earlier termination, and shorter period of growing season for the pioneer tree species (birch and aspen) than the dominant tree species (larch). Root phenology showed later initiation, later peak and later termination dates than leaf phenology. An asynchronous correlation of R a and radial growth was identified with a time lag of approximately 1 month, indicating aprioritization of shoot growth. Furthermore, we found that R a was strongly correlated with soil temperature and air temperature, while radial growth was only significantly correlated with air temperature, implying a down-regulating effect of temperature. Our results indicate different phenologies between pioneer and dominant species and support a down-regulation hypothesis of plant phenology which can be helpful in understanding forest dynamics in the context of climate change.






Similar content being viewed by others
References
Aloni R (2001) Foliar and axial aspects of vascular differentiation: hypotheses and evidence. J Plant Growth Regul 20:22–34
Aloni R (2013) Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation. Planta 238(5):819–830
Alvarez-Uria P, Körner C (2007) Low temperature limits of root growth in deciduous and evergreen temperate tree species. Funct Ecol 21:211–218
Bond-Lamberty B, Thomson A (2010) A global database of soil respiration data. Biogeosciences 7(6):1915–1926
Burke MK, Raynal DJ (1994) Fine root growth phenology, production, and turnover in a northern hardwood forest ecosystem. Plant Soil 162(1):135–146
Cannell MGR (1989) Physiological basis of wood production: a review. Scand J For Res 4(1–4):459–490
Chmielewski FM, Rötzer T (2001) Response of tree phenology to climate change across Europe. Agr For Meteorol 108:101–112
Chuine I (2010) Why does phenology drive species distribution? Philos Trans R Soc B 365:3149–3160
Côté B, Hendershot WH, Fyles JW, Roy AG, Bradley R, Biron PM, Courchesne F (1998) The phenology of fine root growth in a maple-dominated ecosystem: relationships with some soil properties. Plant Soil 201(1):59–69
Desrochers A, Landhausser SM, Lieffers VJ (2002) Coarse and fine root respiration in aspen (Populus tremuloides). Tree Physiol 22:725–732
Ding Y, Wang Z, Sun Y (2008) Inter-decadal variation of the summer precipitation in East China and its association with decreasing Asian summer monsoon. Part I: Observed evidences. Int J Climatol 28(9):1139–1161
Du EZ, Zhou Z, Li P, Jiang L, Hu XY, Fang JY (2013) Winter soil respiration during soil freezing in a boreal forest in Northeast China. J Plant Ecol 6:349–357
Finegan B (1984) Forest succession. Nature 312(8):109–114
Gaudinski JB, Torn MS, Riley WJ et al (2009) Use of stored carbon reserves in growth of temperate tree roots and leaf buds: analyses using radiocarbon measurements and modeling. Global Change Biol 15(4):992–1014
Gordo O, Sanz JJ (2010) Impact of climate change on plant phenology in Mediterranean ecosystems. Global Change Biol 16:1082–1106
Harris GA (1977) Root phenology as a factor of competition among grass seedlings. J Range Manage 30(30):172–177
Häsler R, Streule A, Turner H (1999) Shoot and root growth of young Larix decidua in contrasting microenvironments near the alpine timberline. Phyton 39:47–52
Hendrick RL, Pregitzer KS (1992) The demography of fine roots in a northern hardwood forest. Ecology 73:1094–1104
Hoad GV (1995) Transport of hormones in the phloem of higher plants. Plant Growth Regul 16(2):173–182
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Högberg MN, Nyberg G, Ottosson-Löfvenius M, Readk DJ (2001) Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–792
Ide R, Oguma H (2010) Use of digital cameras for phenological observations. Ecol Inform 5:339–347
Jackson RB, Lechowicz MJ, Li X, Mooney HA (2001) Phenology, growth, and allocation in global terrestrial productivity. In: Roy J, Saugier B, Mooney HA (eds) Terrestrial global productivity. Academic Press, San Diego, pp 61–82
Jeong SJ, Ho CH, Gim HJ, Brown ME (2011) Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Global Change Biol 17:2385–2399
Jiang HY, Gu JC, Qiu J, Wang ZQ (2010) Seasonal variations of fine root production and mortality in Larix gmelinii plantation in 2004–2008. Chin J Appl Ecol 21(10):2465–2471
Johnson MG, Tingey DT, Phillips DL et al (2001) Advancing fine root research with minirhizotrons. Environ Exp Bot 45:263–289
Joslin JD, Wolfe MH, Hanson PJ (2001) Factors controlling the timing of root elongation intensity in a mature upland oak stand. Plant Soil 228(2):201–212
Körner C (2012) Alpine treelines: functional ecology of the global high elevation tree limits. Springer, Berlin
Körner C, Basler D (2010) Phenology under global warming. Science 327:1461–1462
Kossuth SV, Ross SD (1987) Hormonal control of tree growth. Plant Growth Regul 6:1–215
Kuzyakov Y, Gavrichkova O (2010) REVIEW: time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls. Global Change Biol 16(12):3386–3406
Landhäusser SM, Lieffers VJ (2003) Seasonal changes in carbohydrate reserves in mature northern Populus tremuloides clones. Trees Struct Funct 17:471–476
Menzel A (2002) Phenology: its importance to the global change community. Clim Change 54:379–385
Milchunas DG (2009) Estimating root production: comparison of 11 methods in shortgrass steppe and review of biases. Ecosystems 12(8):1381–1402
Nord EA, Lynch JP (2009) Plant phenology: a critical controller of soil resource acquisition. J Exp Bot 60:1927–1937
Palacio S, Montserrat-Martí G (2007) Above and belowground phenology of four Mediterranean sub-shrubs. Preliminary results on root–shoot competition. J Arid Environ 68:522–533
Piao SL, Ciais P, Huang Y et al (2010) The impacts of climate change on water resources and agriculture in China. Nature 467:43–51
Polgar CA, Primack RB (2011) Leaf-out phenology of temperate woody plants: from trees to ecosystems. New Phytol 191(4):926–941
Quan X, Wang C, Zhang Q, Wang X, Luo Y, Bond-Lamberty B (2010) Dynamics of fine roots in five Chinese temperate forests. J Plant Res 123(4):497–507
Richardson AD, Black TA, Ciais P et al (2010) Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos Trans R Soc B 365:3227–3246
Schenker G, Lenz A, Körner C, Hoch G (2014) Physiological minimum temperatures for root growth in seven common European broad-leaved tree species. Tree Physiol 34:302–313
Steinaker DF, Wilson SD (2008) Phenology of fine roots and leaves in forest and grassland. J Ecol 96(6):1222–1229
Steinaker DF, Wilson SD, Peltzer DA (2010) Asynchronicity in root and shoot phenology in grasses and woody plants. Global Change Biol 16:2241–2251
Tierney GL, Fahey TJ, Groffman PM, Hardy JP, Fitzhugh RD, Driscoll CT, Yavitt JB (2003) Environmental control of fine root dynamics in a northern hardwood forest. Global Change Biol 9:670–679
Tryon PR, Chapin FS III (1983) Temperature control over root growth and root biomass in taiga forest trees. Can J For Res 13(5):827–833
Veen BW (1981) Relation between root respiration and root activity. Plant Soil 63(1):73–76
Wan XC, Landhäusser SM, Zwiazek JJ, Lieffers VJ (1999) Root water flow and growth of aspen (Populus tremuloides) at low root temperatures. Tree Physiol 19:879–884
Wilson JB (1988) A review of evidence on the control of shoot:root ratio, in relation to models. Ann Bot 61:433–449
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 31021001), the National Basic Research Program of China on Global Change (2010CB950600), and the Ministry of Science and Technology (2010DFA31290). We are grateful to Dr Lucy Sheppard (Centre for Ecology and Hydrology, UK) for her help with linguistic editing of the manuscript. Neither author has a conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Russell Monson.
Rights and permissions
About this article
Cite this article
Du, E., Fang, J. Linking belowground and aboveground phenology in two boreal forests in Northeast China. Oecologia 176, 883–892 (2014). https://doi.org/10.1007/s00442-014-3055-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3055-y